What temperature does thermometer indicate The temperature hovers





























- Slides: 36


What temperature does thermometer indicate?

The temperature hovers at zero Celsius.

Essential Question ¡Understanding how does heat affect a system?

What does it mean to have a temperature of 0 C? What is temperature? Is temperature the same thing as heat?

So what is heat? ¡ Heat is the amount of thermal energy. For example, the sparks from a sparkler are at around 800°C but do not burn your skin. However, a hot cup of tea at around 100°C will burn your hand badly. This is because the tea contains more heat energy, even though it is cooler.

Temperature is a measure of how “hot” or “cold” something is. Temperature is measured in arbitrary units, like Fahrenheit or Celsius.

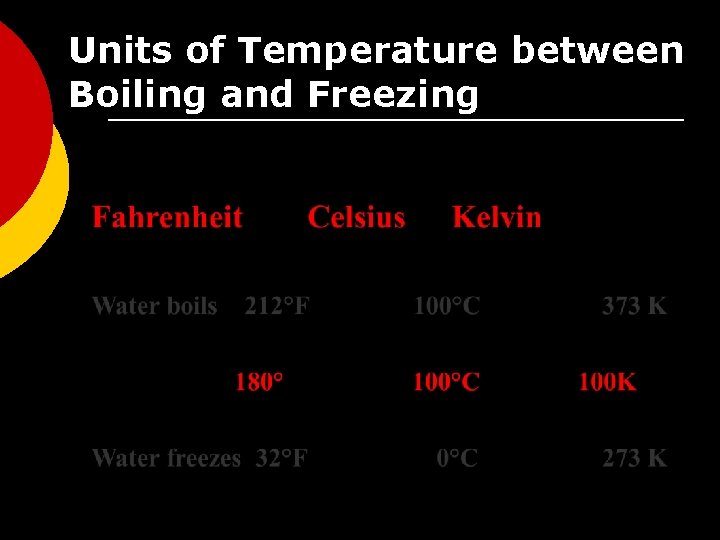
Units of Temperature between Boiling and Freezing

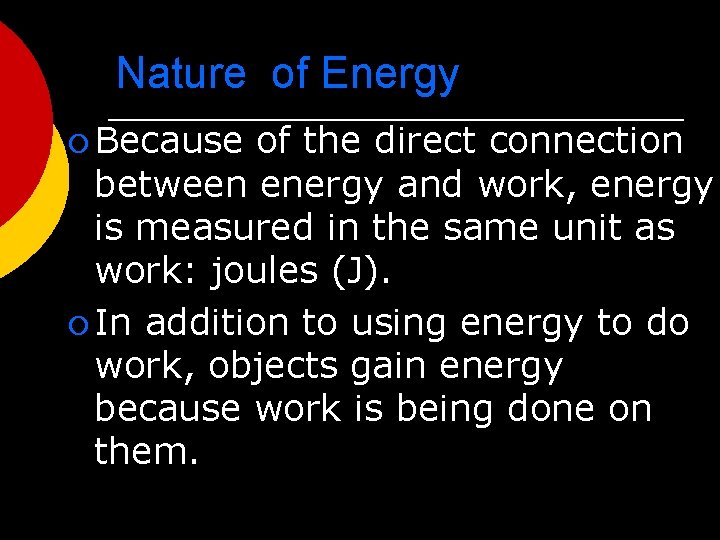
Nature of Energy ¡ Energy is all around you! l You can hear energy as sound. l You can see energy as light. l And you can feel it as wind.

Nature of Energy ¡ What is energy that it can be involved in so many different activities? l Energy can be defined as the ability to do work. l If an object or organism does work (exerts a force over a distance to move an object) the object or organism uses energy.

Forms of Energy ¡The five main forms of energy are: l Heat l Chemical l Electromagnetic l Nuclear l Mechanical

Nature of Energy ¡ Because of the direct connection between energy and work, energy is measured in the same unit as work: joules (J). ¡ In addition to using energy to do work, objects gain energy because work is being done on them.

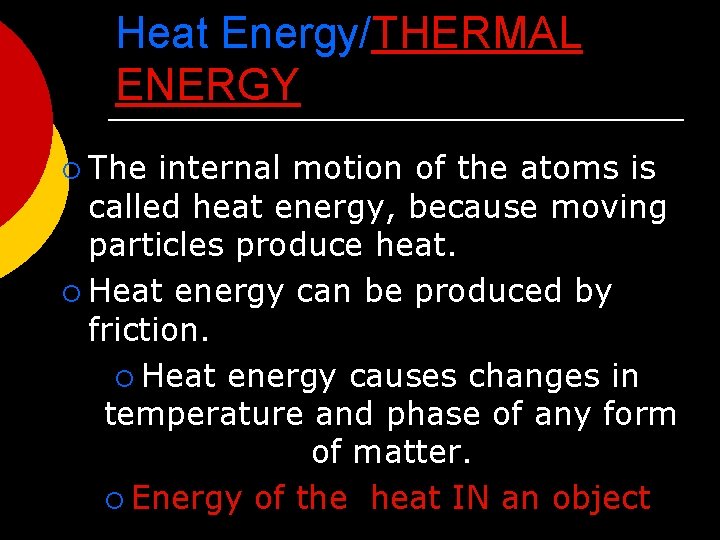
Heat Energy/THERMAL ENERGY ¡ The internal motion of the atoms is called heat energy, because moving particles produce heat. ¡ Heat energy can be produced by friction. ¡ Heat energy causes changes in temperature and phase of any form of matter. ¡ Energy of the heat IN an object

States of Energy ¡ The most common energy conversion is the conversion between potential and kinetic energy. ¡ All forms of energy can be in either of two states: l Potential l Kinetic

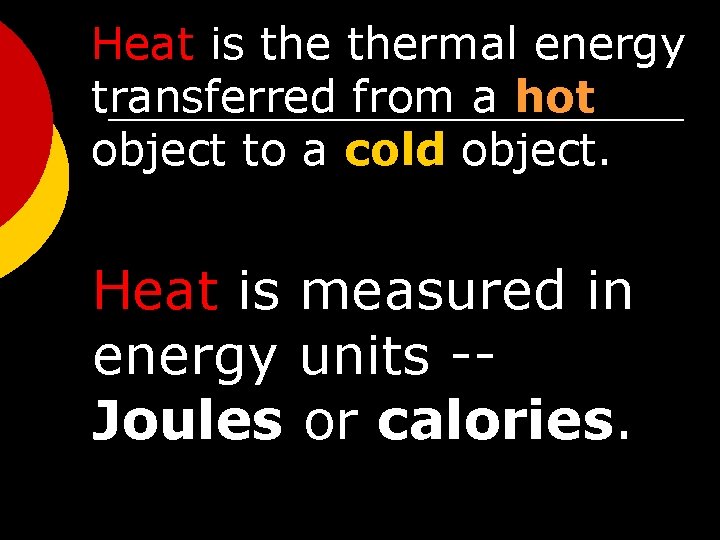
Heat is thermal energy transferred from a hot object to a cold object. Heat is measured in energy units -Joules or calories.

Is heat absorbed or released during a phase change?

We know that heat is either absorbed or released during phase change. Heat is absorbed as solids melt, or liquids vaporize.

Heat is released as liquids freeze, or vapors condense.

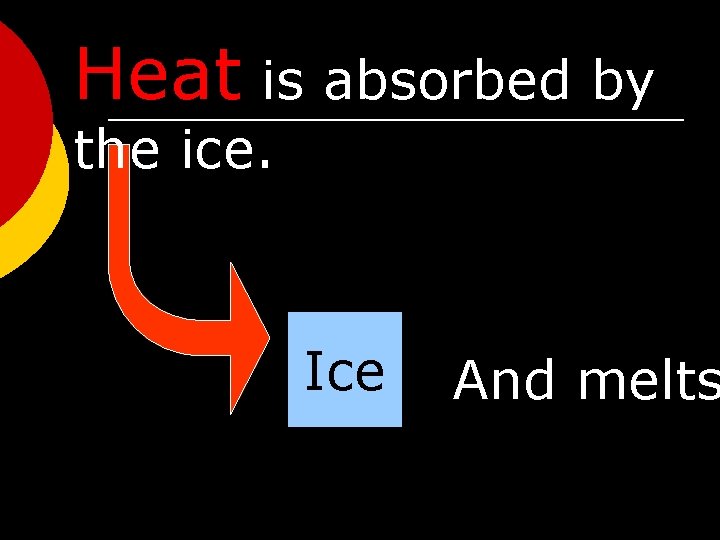
Heat is absorbed by the ice. Ice And melts

Heat is absorbed by the ice. One gram of ice at 0 C absorbs 334 J as it melts to form water at 0 C. … making liquid water

Heat is released by the water as it freezes. 334 joules is Ice water released when one gram of water freezes at 0 C.

Ice absorbs 334 J per gram as it melts at 0 C Ice Water releases 334 J per gram as it freezes at 0 C

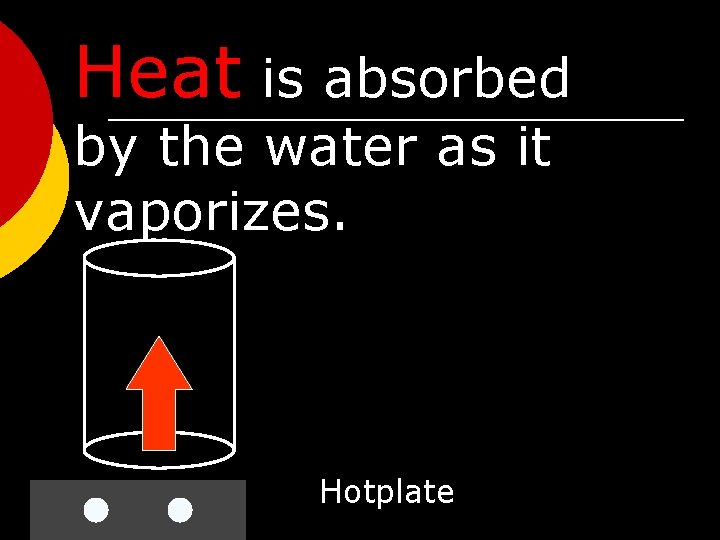
Heat is absorbed by the water as it vaporizes. Hotplate

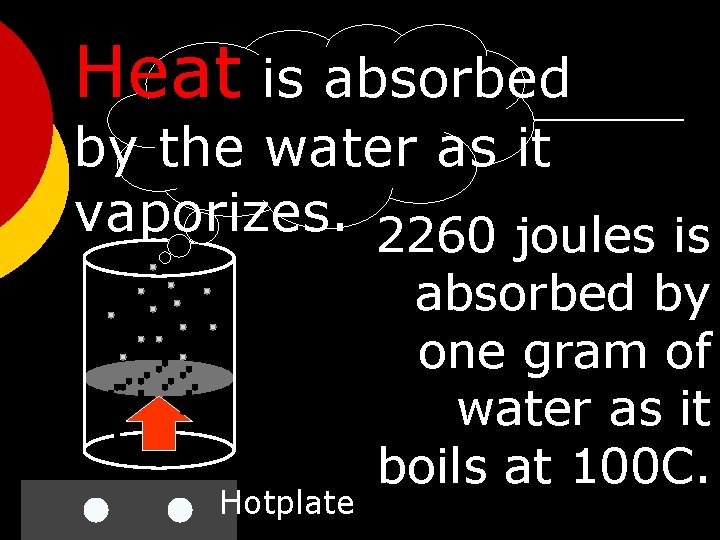
Heat is absorbed by the water as it vaporizes. 2260 joules is Hotplate absorbed by one gram of water as it boils at 100 C.

Steam releases 2260 J/g as it condens es at 100 C Water absorbs 2260 J/g as it boils at 100 C Hotplate

Heat is released by water vapor as it condenses.

Heat is released by water vapor as it condenses. The heat released by condensing water vapor is a major factor in weather phenomena like thunderstorms and

Phase changes occur at a constant temperature as heat is absorbed or

Question for discussion: If phase changes occur at a constant temperature, then what happens to the heat when water boils?

The heat energy breaks the intermolecular bonds which keep the water in the liquid phase.

States of Energy: Kinetic and Potential Energy ¡Kinetic Energy is the energy of motion. ¡Potential Energy is stored energy.

Kinetic Energy The energy of motion is called kinetic energy. ¡ The faster an object moves, the more kinetic energy it has. ¡ The greater the mass of a moving object, the more kinetic energy it has. ¡ Kinetic energy depends on both mass and velocity. ¡

Potential Energy ¡ Potential Energy is stored energy. l Stored chemically in fuel, the nucleus of atom, and in foods. l Or stored because of the work done on it: ¡ Stretching a rubber band. ¡ Winding a watch. ¡ Pulling back on a bow’s arrow. ¡ Lifting a brick high in the air.

Gravitational Potential Energy ¡ Potential energy that is dependent on height is called gravitational potential energy.

Potential Energy ¡ Energy that is stored due to being stretched or compressed is called elastic potential energy.

The Law of Conservation of Energy ¡ Energy can be neither created nor destroyed by ordinary means. l l It can only be converted from one form to another. If energy seems to disappear, then scientists look for it – leading to many important discoveries. All images used are from Microsoft Office ©Copyright 2014 -all rights reserved www. cpalms. org