2012 Cidex OPA GUIDELINES Standardizing Cidex OPA Use





















- Slides: 32

2012 Cidex® OPA GUIDELINES Standardizing Cidex® OPA Use For Direct Users Tia Gonnella 4/1/12

This Module: � This module is applicable to direct users of Cidex OPA® Tia Gonnella 4/1/12

Purpose � To ensure correct Cidex® OPA guidelines are followed: � OSHA � Manufacturer � Infection guidelines Control � Safety � Compliance Tia Gonnella 4/1/12

Objectives ØTo Standardize the use of Cidex® OPA within the system including: Safe Use Labeling Testing Soaking Documentation Spill Protocol Tia Gonnella 4/1/12

Safety Personal Protective Equipment Ø Always OPA use PPE when handling Cidex® Placing probe into Cidex OPA®soak container Removing probe from soak container Testing solution/strips Changing solution Tia Gonnella 4/1/12

Cidex OPA® Stain Cidex bottles were laying on side in drawer when one leaked. These stains are permanent and can occur on clothing, skin, nails, hair, transducers, or any contact surface. This is one of the things PPE protects against. Tia Gonnella 4/1/12

Cidex OPA® Stain in drawer Cidex OPA® leaked inside drawer staining surface. Tia Gonnella 4/1/12

Safety (cont. ) � Ventilation ◦ GUS station � Location of soak container ◦ Low traffic area/secured from public access ◦ Well ventilated room ◦ Labeled containers Tia Gonnella 4/1/12

Opening Cidex® OPA � PPE ◦ Gloves, gown, mask, eye protection � Neutralize spilled solution with Glute-Out® ◦ Wait five minutes before pouring down drain, allow water to run for a few minutes ◦ You do not have to neutralize unused solution in container prior to disposal (spills only) � Rinse containers until water runs clear ◦ Cap and dispose of old bottle in trash Tia Gonnella 4/1/12

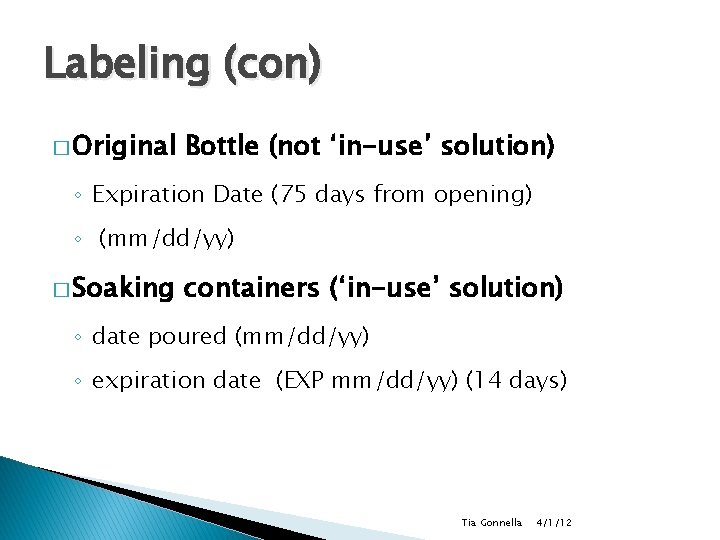
Labeling (con) � Original Bottle (not ‘in-use’ solution) ◦ Expiration Date (75 days from opening) ◦ (mm/dd/yy) � Soaking containers (‘in-use’ solution) ◦ date poured (mm/dd/yy) ◦ expiration date (EXP mm/dd/yy) (14 days) Tia Gonnella 4/1/12

Labeling � Secondary soaking containers ◦ Must be labeled “Cidex® OPA” ◦ A warning sign must be visible to patients ◦ An additional label must indicate it is a hazardous chemical Obtained through Central Supply A thermometer must be used to obtain accurate solution temperature Tia Gonnella 4/1/12

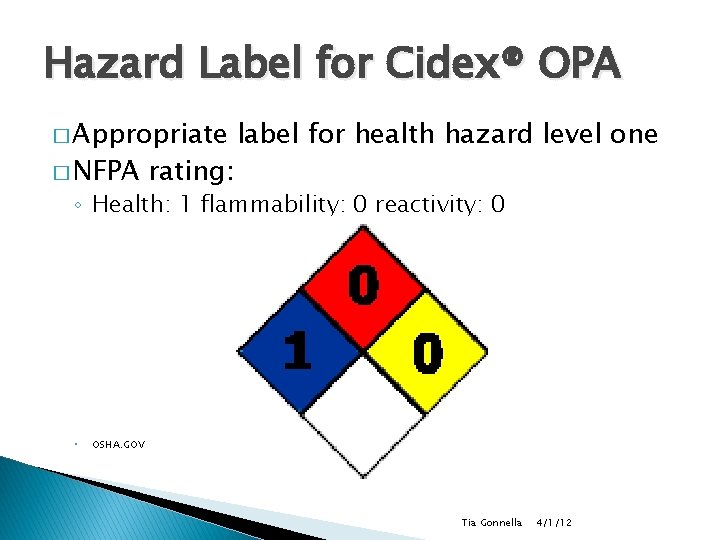
Hazard Label for Cidex® OPA � Appropriate � NFPA rating: label for health hazard level one ◦ Health: 1 flammability: 0 reactivity: 0 ◦ OSHA. GOV Tia Gonnella 4/1/12

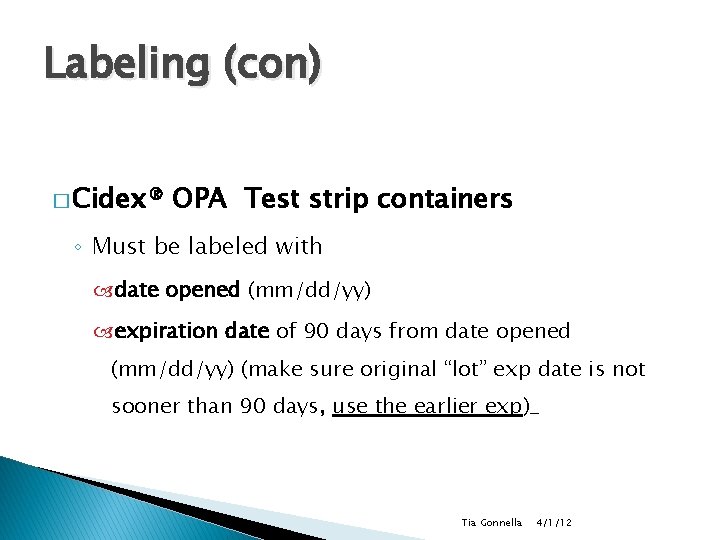
Labeling (con) � Cidex® OPA Test strip containers ◦ Must be labeled with date opened (mm/dd/yy) expiration date of 90 days from date opened (mm/dd/yy) (make sure original “lot” exp date is not sooner than 90 days, use the earlier exp)_ Tia Gonnella 4/1/12

Testing � Cidex® OPA solution must be tested: ◦ Every new bottle of solution opened With 3 full strength and 3 controls (half filled with cidex half filled with water ) ◦ Before soaking probe (every time) � Test strips must be tested: ◦ each new bottle opened ◦ Weekly QA (3 full strength/3 controls) Tia Gonnella 4/1/12

How to Use Test Strips � Opening ◦ Mark the date on the bottle in space provided ◦ Mark the expiration date (90 days from date of opening) in space provided � Perform control test for ◦ each new bottle opened ◦ On a weekly QA basis Tia Gonnella 4/1/12

Testing Cidex® OPA with Test Strips � Submerge test strip in solution for one second, do not shake or swirl � Remove excess solution by standing the strip upright on a paper towel. � Read results at 90 seconds. ◦ Do not read past 90 seconds the results will be invalid. Pad will be completely purple to indicate effective solution. If any blue remains on the pad, the solution is ineffective and must be discarded. � Advanced sterilization products (2012) retrieved 7/26/2012 from http: //www. aspjj. com/us/products/cidexopa/features-and-benefits Tia Gonnella 4/1/12

Test Strips (con) � Results: � Advanced sterilization products (2012) retrieved 7/26/2012 from http: //www. aspjj. com/us/products/cidexopa/features-and-benefits Tia Gonnella 4/1/12

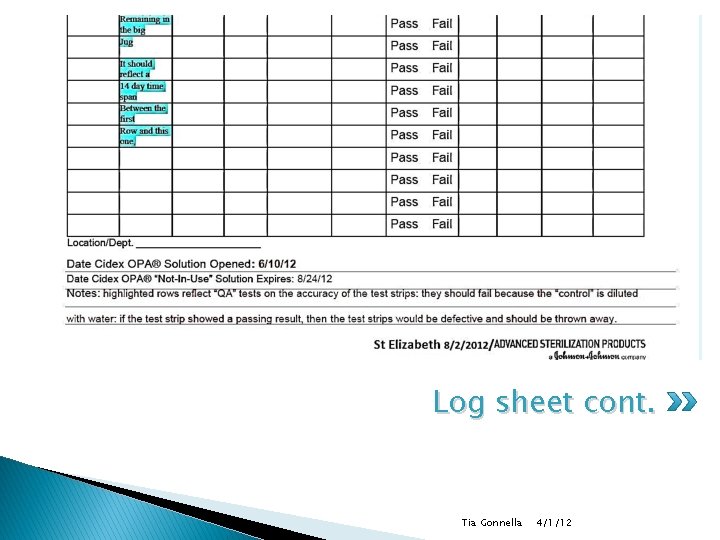
Test strips (con) � Follow same instructions for 3 full strength cidex tests and 3 controls (half water half cidex) ◦ Test strip should fail for control/mark control in comment field ◦ Highlight row on log sheet for QA tests � Tightly recap test strip bottle after each use. � Discard any unused test strips 90 days after bottle is originally opened. Tia Gonnella 4/1/12

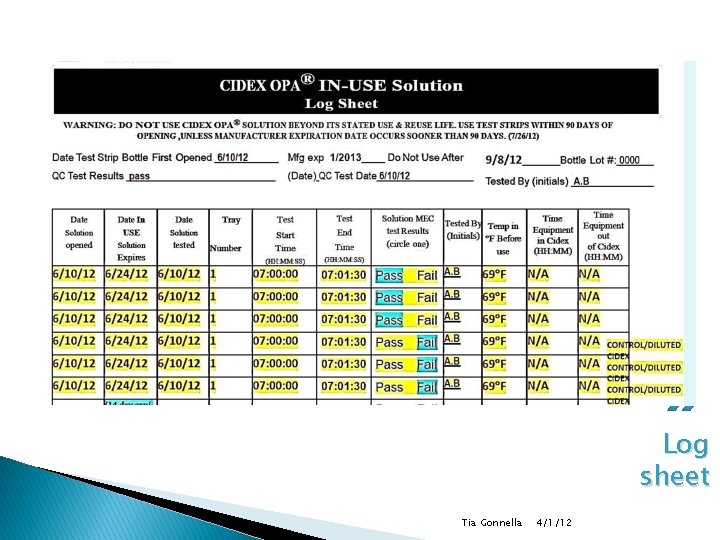
Testing Documentation ◦ Record in log book Date (mm/dd/yy) Date solution activated/expired Date test strips expire Date of test time of test start (hh: mm: ss) time of test end (hh: mm: ss) Must include seconds to demonstrate compliance temperature of solution results (pass or fail) Time equipment placed in solution/taken out of solution (hh: mm) Tia Gonnella 4/1/12

Soaking � Must have submersible thermometer to test temperature of solution � SOLUTION TEMPERATURE ◦ Cidex® OPA solution must be at least 20 C/68 F to be effective Tia Gonnella 4/1/12

Soaking � Thoroughly remove gel from probe ◦ Use sani-wipe/alternative to remove proteins dry � Insert probe into solution-use clip to hold transducer to avoid breakage of scan head � 12 minutes to high-level disinfect � Must be removed after 12 minutes (use timer) � Mark on log sheet time in/time out of solution Tia Gonnella 4/1/12

Rinsing � Before soak: ◦ Test solution ◦ Thoroughly remove gel from probe ◦ Use Sani-wipe/alternative to remove protein ◦ Dry probe prior to soak Tia Gonnella 4/1/12

Rinsing � After Soaking in Cidex OPA® � Remove � Wait probe from solution place in fresh water one minute, remove (place on towel) ◦ Replace water ◦ Repeat two additional times Three separate one-minute soaks in fresh potable water WATER MUST BE CHANGED AFTER EACH ONE MINUTE SOAK Dispose of final rinse water after third rinse—do not leave standing water in soak container Tia Gonnella 4/1/12

Documentation � Use Cidex® OPA Log Sheets � Documentation required for ◦ Cidex® OPA solution control test performed on each newly opened bottle ◦ Cidex® OPA test strips control test 3/3 for each new bottle of test strips opened ◦ Cidex® OPA solution -test prior to soaking probe (after every pelvic patient) ◦ QA weekly control test on test strips Tia Gonnella 4/1/12

Documentation (con) � Must include: ◦ Container number (where applicable) ◦ Date of solution poured into container (mm/dd/yy) ◦ Date of soak container solution expiration (mm/dd/yy) 14 days ◦ Expiration date of test strips (mm/dd/yy) ◦ Date of test (mm/dd/yy) Tia Gonnella 4/1/12

Documentation (con) ◦ Temperature of solution ◦ Time test started (HH: MM: SS) ◦ Time test ended (HH: MM: SS) ◦ Results ◦ Initials of person testing ◦ Time equipment placed in solution/time out (HH: MM) Tia Gonnella 4/1/12

Log sheet Tia Gonnella 4/1/12

Log sheet cont. Tia Gonnella 4/1/12

Changing Solution � PPE (gown, gloves, mask, and eye protectant) � No deactivation required (spills only) ◦ Dispose down sink drain with water running ◦ Rinse original container until water runs clear and discard Tia Gonnella 4/1/12

Documents � All Cidex OPA® documents are available for technologists’ access in MEDEX under Cidex OPA® Packet ◦ ◦ ◦ Log sheets Competency forms Guidelines for use/spill clean up/QA testing Procedures Safety information Tia Gonnella 4/1/12

Changing Solution (cont) � Refill solution to fill line � Label Containers � Test Solution Tia Gonnella 4/1/12

References OSHA (2006) Best Practices for the Safe Use of Glutaraldehyde in Health Care. Retrieved from www. osha. gov/Publications/glutaraldehyde. pdf Tia Gonnella 4/1/12