WELCOME College name vivekananda college of arts and





























- Slides: 43

WELCOME College name: vivekananda college of arts and science for women-sirkali Staff name : Dr. S. Sasikala Designation : Assistant professor Department : Chemistry Subject : Physical Chemistry-II Subject code : 16 SCCCH 9 Topic : Spectroscopy(UV, IR, NMR)

SPECTROSCOPY


Types of spectroscopy • The nature of the interaction between radiation and matter may include • 1. Absorption • 2. Emission • 3. Scattering

Absorption spectroscopy • In absorption spectroscopy an electromagnetic radiation is absorbed by an atom or molecule Which undergoes transition from a lower energy state to a higher energy or excited state • Absorption occurs only when the energy of radiation matches the difference in energy between two energy levels

Emission spectroscopy • Atoms or molecules that are excited to high energy levels can decay to lower levels by emitting radiation • The substance first absorbs energy and then emits this energy as light • Emission can be induced by sources of energy such as flame or electromagnetic radiation

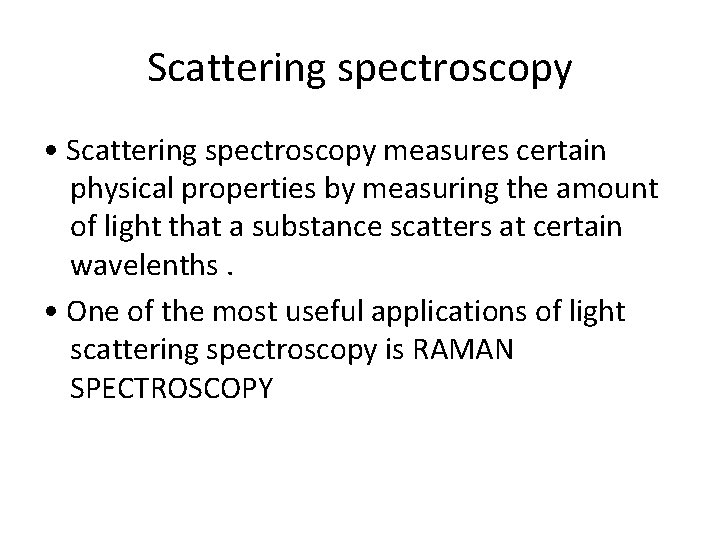
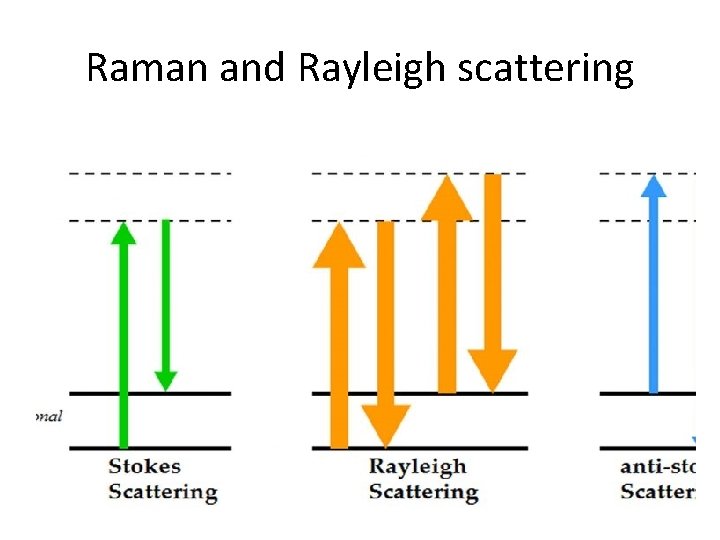
Scattering spectroscopy • Scattering spectroscopy measures certain physical properties by measuring the amount of light that a substance scatters at certain wavelenths. • One of the most useful applications of light scattering spectroscopy is RAMAN SPECTROSCOPY








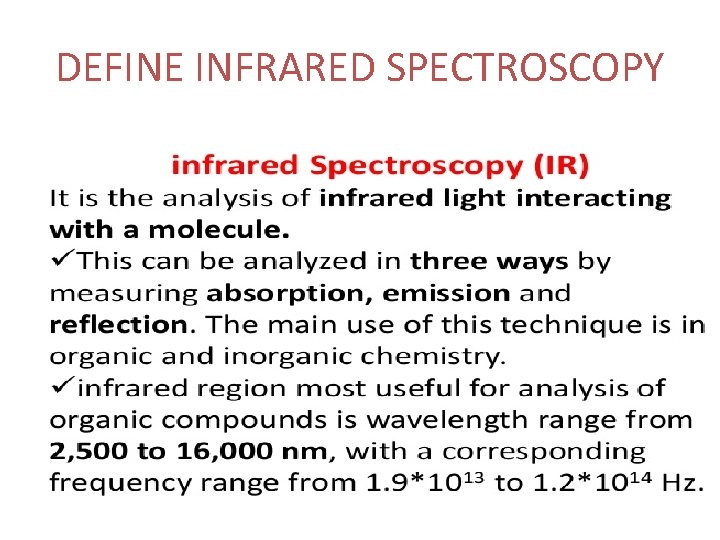
DEFINE INFRARED SPECTROSCOPY


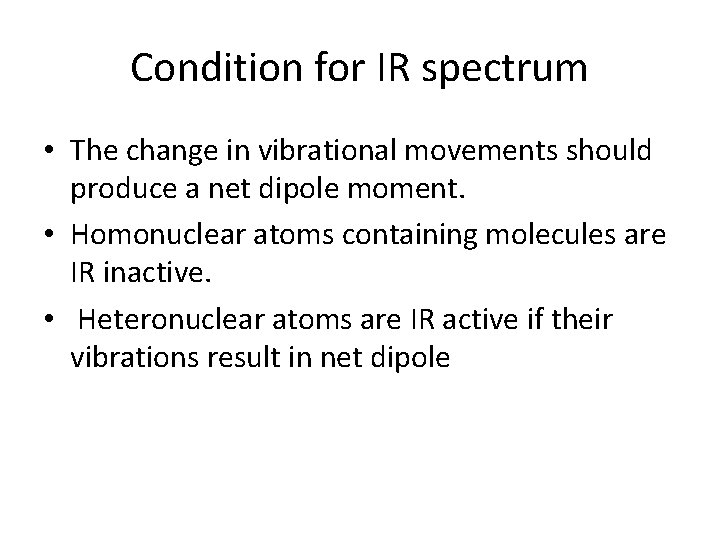
Condition for IR spectrum • The change in vibrational movements should produce a net dipole moment. • Homonuclear atoms containing molecules are IR inactive. • Heteronuclear atoms are IR active if their vibrations result in net dipole

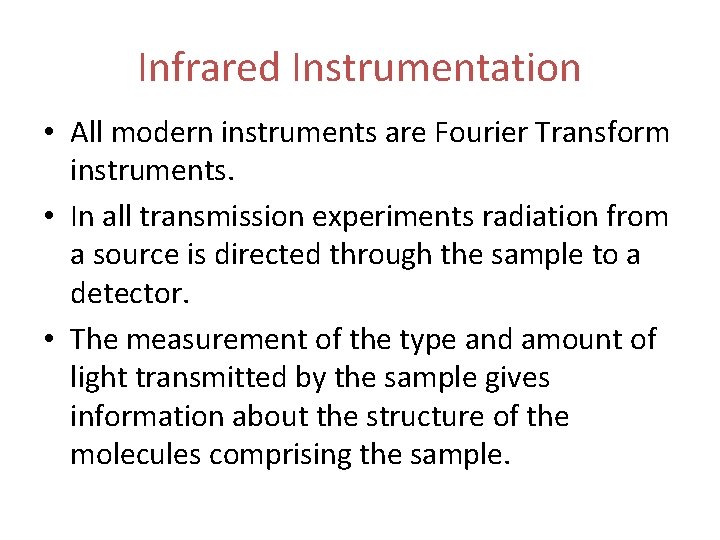
Infrared Instrumentation • All modern instruments are Fourier Transform instruments. • In all transmission experiments radiation from a source is directed through the sample to a detector. • The measurement of the type and amount of light transmitted by the sample gives information about the structure of the molecules comprising the sample.


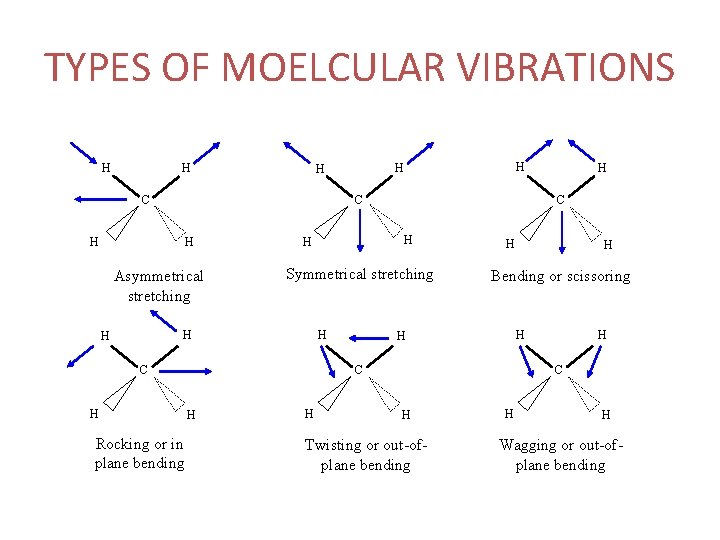
TYPES OF MOELCULAR VIBRATIONS • Stretching: Change in inter-atomic distance along bond axis • Bending: Change in angle between two bonds. There are four types of bend: • Rocking • Scissoring • Wagging • Twisting


TYPES OF MOELCULAR VIBRATIONS H H C H Asymmetrical stretching Symmetrical stretching H H C H Rocking or in plane bending H H H Bending or scissoring H H C C H H H C H Twisting or out-ofplane bending H H Wagging or out-ofplane bending

overtones § Bands corresponding to integral multiple of fundamental vibration. § They are due to transition from ground state to higher vibrational states. § They are very weak bands. § An absorption band at 1050 cm-1 may well have an accompanying band at 2100 (2 ν) and 3150 (3 ν) cm-1.

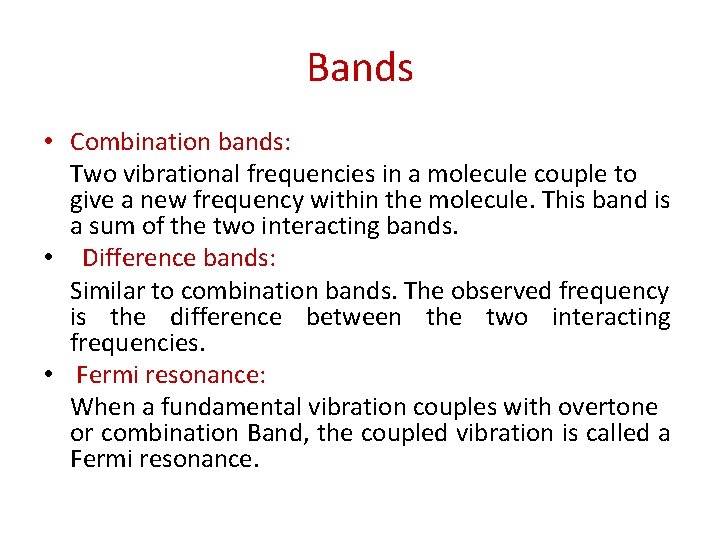
Bands • Combination bands: Two vibrational frequencies in a molecule couple to give a new frequency within the molecule. This band is a sum of the two interacting bands. • Difference bands: Similar to combination bands. The observed frequency is the difference between the two interacting frequencies. • Fermi resonance: When a fundamental vibration couples with overtone or combination Band, the coupled vibration is called a Fermi resonance.


Raman Spectroscopy


Raman and Rayleigh scattering










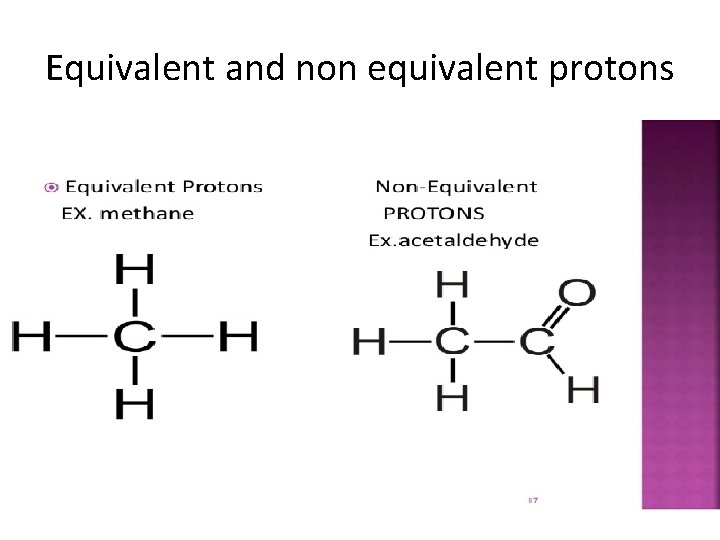
Equivalent and non equivalent protons




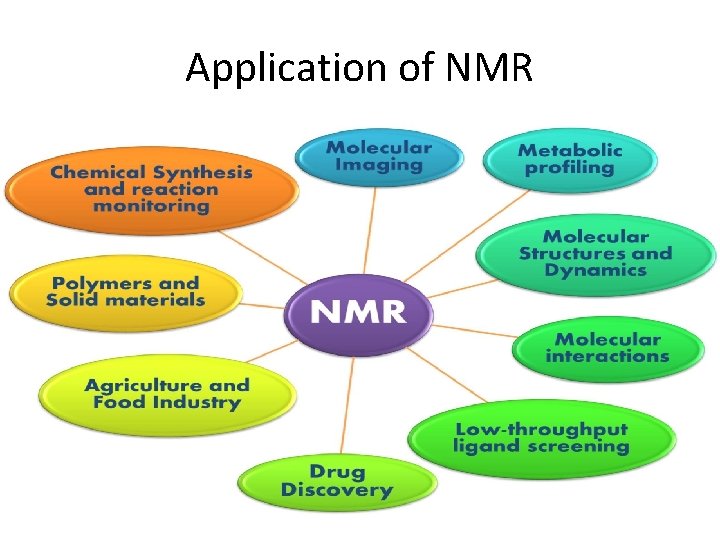
Application of NMR

THANK YOU
 Swami vivekananda and tesla
Swami vivekananda and tesla Define
Define Swami vivekananda ap world history
Swami vivekananda ap world history Creative arts grade 8 lesson plans term 2
Creative arts grade 8 lesson plans term 2 Queens college of arts and science
Queens college of arts and science Queens college
Queens college Bon secours college mannargudi courses
Bon secours college mannargudi courses Dorothy f schmidt college of arts and letters
Dorothy f schmidt college of arts and letters Ves college of arts science and commerce
Ves college of arts science and commerce Uf clas advising
Uf clas advising Draw three noncollinear points j k and l
Draw three noncollinear points j k and l Welcome welcome this is our christmas story
Welcome welcome this is our christmas story Lahc verify my fafsa
Lahc verify my fafsa Ilfracombe arts college
Ilfracombe arts college Valeriaim of
Valeriaim of Welcome back name
Welcome back name Jocelyn kirsch
Jocelyn kirsch Authors last name first name initial
Authors last name first name initial Stock and classical names of elements
Stock and classical names of elements Kyiv national university of culture and arts
Kyiv national university of culture and arts Academy of motion picture arts and sciences benefits
Academy of motion picture arts and sciences benefits Hawaii academy of arts and science
Hawaii academy of arts and science Ual level 3 performing and production arts
Ual level 3 performing and production arts Atec
Atec Artist or artisan medium and technique
Artist or artisan medium and technique Putnam academy of arts and science
Putnam academy of arts and science Victorian curriculum visual arts scope and sequence
Victorian curriculum visual arts scope and sequence Lfpa warner
Lfpa warner Health and safety in performing arts
Health and safety in performing arts Health and safety in performing arts
Health and safety in performing arts Tagalog script
Tagalog script Columbus humanities arts and technology academy
Columbus humanities arts and technology academy Cal state la arts and letters advising
Cal state la arts and letters advising Arts and crafts movement 1880 to 1910
Arts and crafts movement 1880 to 1910 It is the building blocks of arts and design
It is the building blocks of arts and design Unit 4 music and arts
Unit 4 music and arts What did the pawnee wear
What did the pawnee wear Art culture meaning
Art culture meaning Art and humanities endorsement
Art and humanities endorsement Visual arts and emerging media management
Visual arts and emerging media management George washington carver center for arts and technology
George washington carver center for arts and technology Senior high school strand
Senior high school strand Basic elements of musical play/theatre
Basic elements of musical play/theatre Principles of art
Principles of art