Water Life Civilization Why Water Water H 2









- Slides: 31

Water, Life, Civilization: Why Water?

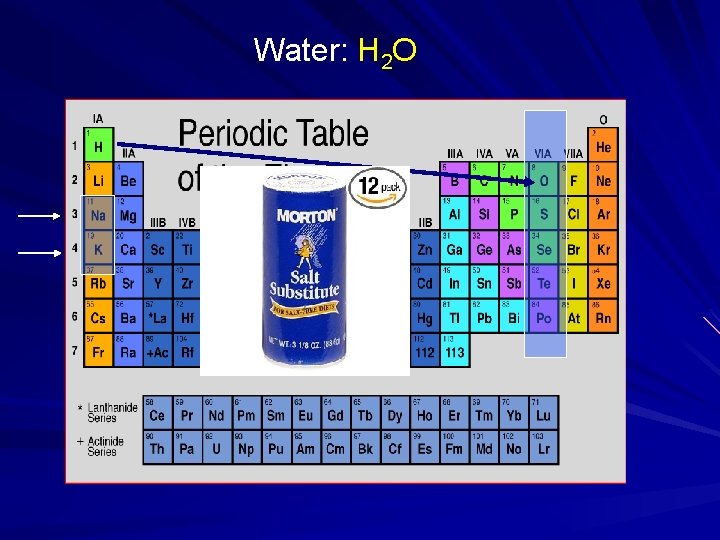
Water: H 2 O

Water: H 2 O

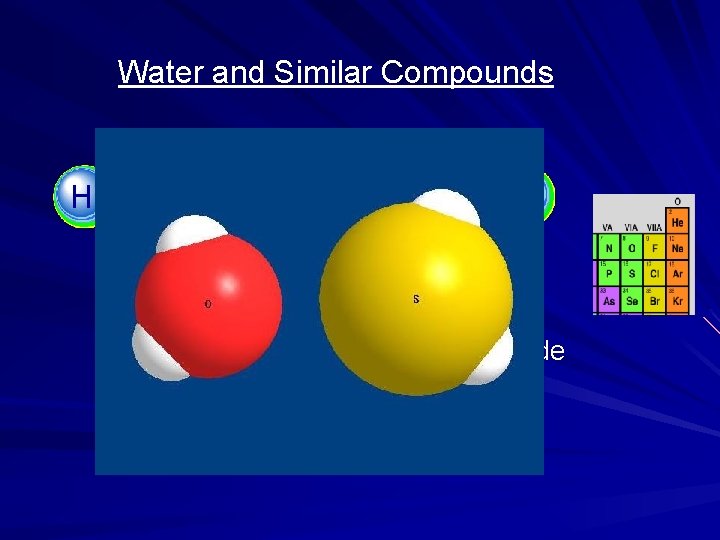
Water and Similar Compounds H H O S Water H 2 O Hydrogen Sulfide H 2 S

Water: H 2 O Hydrogen Sulfide: H 2 S

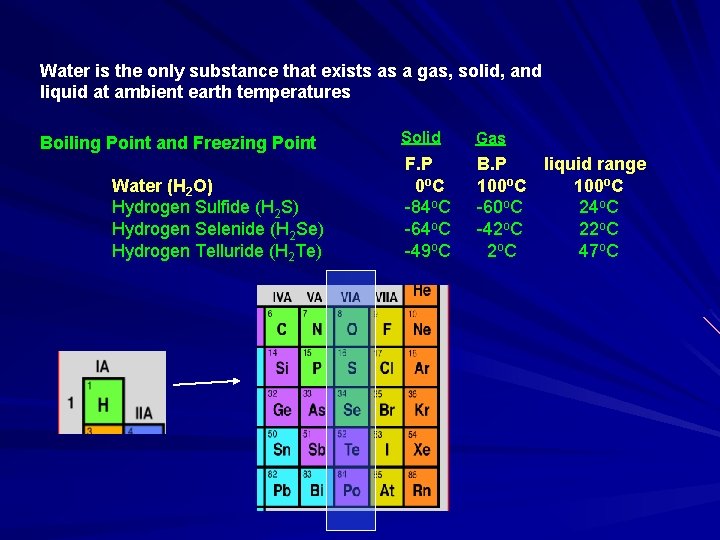
Water is the only substance that exists as a gas, solid, and liquid at ambient earth temperatures Boiling Point and Freezing Point Water (H 2 O) Hydrogen Sulfide (H 2 S) Hydrogen Selenide (H 2 Se) Hydrogen Telluride (H 2 Te) Solid Gas F. P 0 o. C -84 o. C -64 o. C -49 o. C B. P liquid range 100 o. C -60 o. C 24 o. C -42 o. C 2 o. C 47 o. C

H 2 O Two hydrogen atoms One Oxygen atom What makes water so unusual?

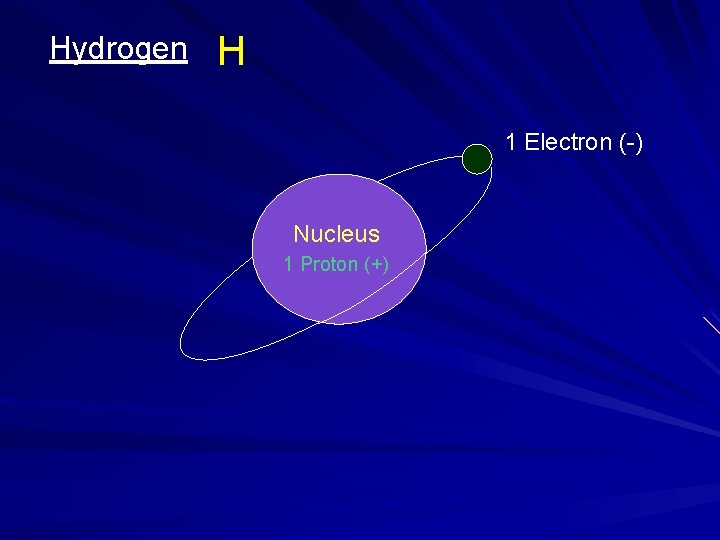
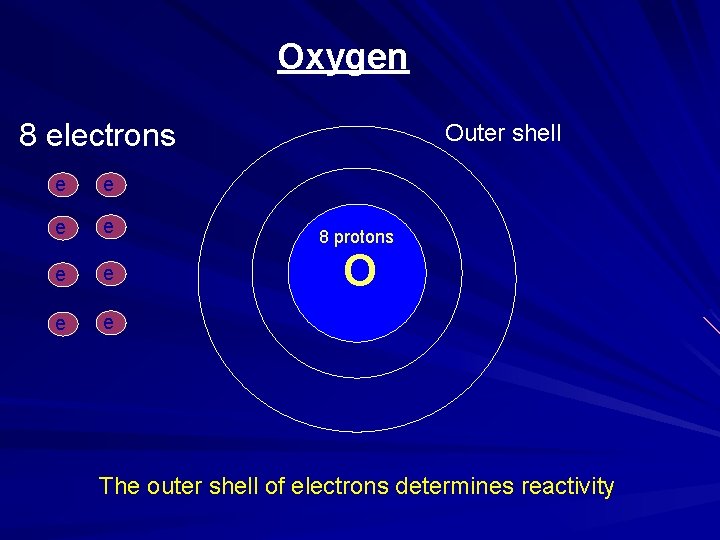
Hydrogen: 1 electron (-), 1 proton (+) Oxygen: 8 electrons (-), 8 protons (+) In water, the hydrogens shares their one electron with oxygen, which shares one of its electrons with each hydrogen. This sharing of electrons forms the bond between hydrogen and oxygen atoms to make the water molecule.

Hydrogen H 1 Electron (-) Nucleus 1 Proton (+)

Oxygen 8 electrons e e e e - - Outer shell - 8 protons O - The outer shell of electrons determines reactivity

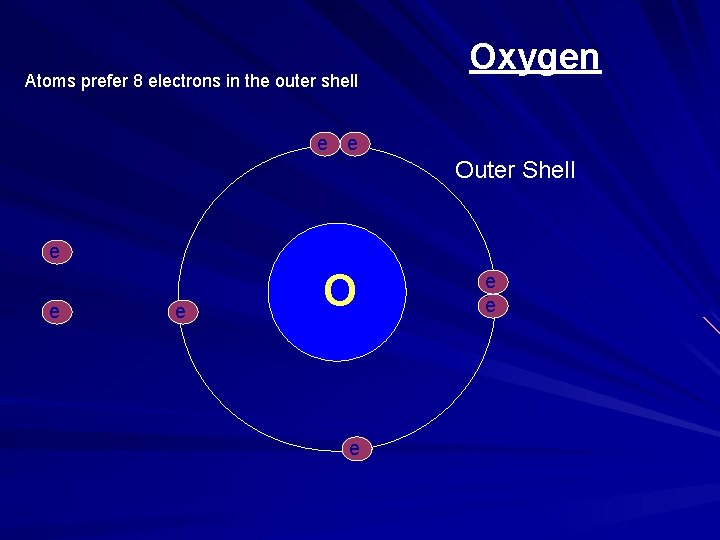
Atoms prefer 8 electrons in the outer shell Oxygen e e - - Outer Shell e - e O - e e -

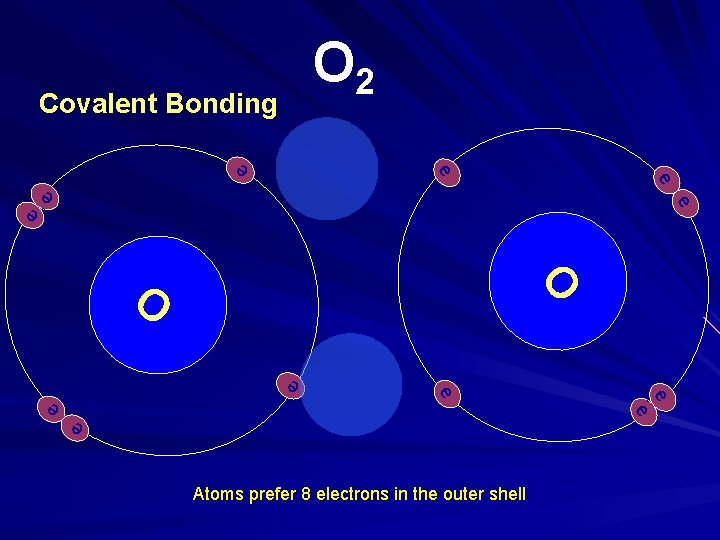
How does oxygen exist in the atmosphere? O 2

O 2 Covalent Bonding e- - e- e - e- O - ee - e- e - Atoms prefer 8 electrons in the outer shell e e e

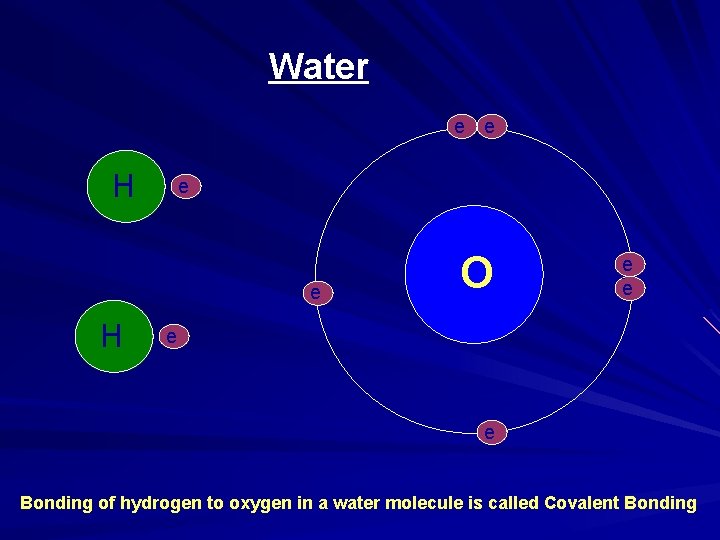
Water e e - HH - e O - H e e - e - Bonding of hydrogen to oxygen in a water molecule is called Covalent Bonding

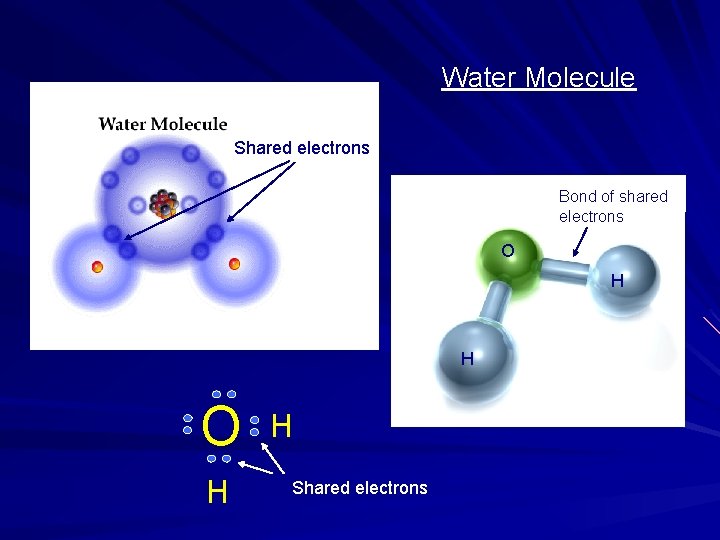
Water Molecule Shared electrons Bond of shared electrons O H H Shared electrons

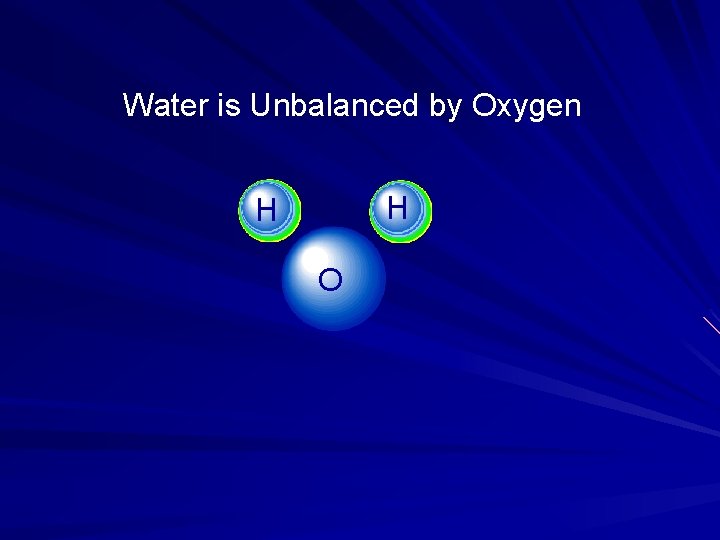
Water is Unbalanced by Oxygen H H O

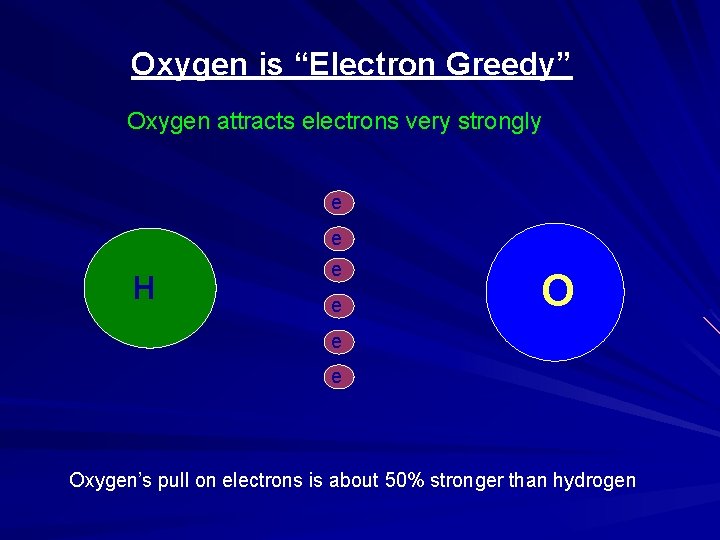
Oxygen is “Electron Greedy” Oxygen attracts electrons very strongly e - H e - e - Oxygen’s pull on electrons is about 50% stronger than hydrogen

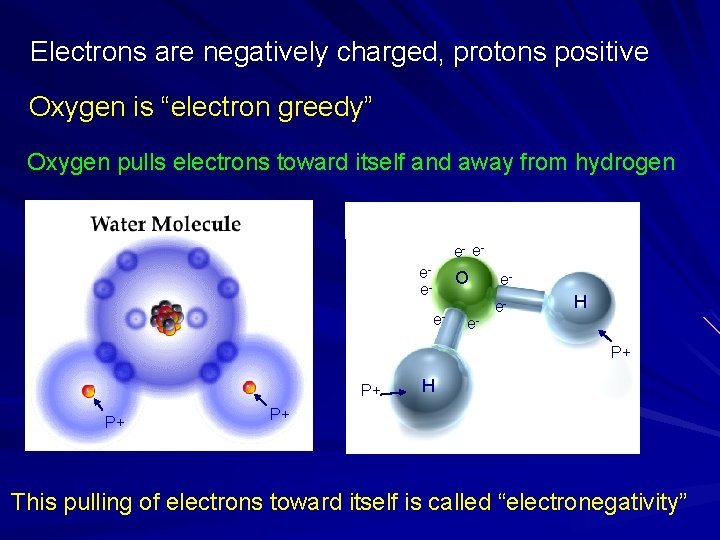
Electrons are negatively charged, protons positive Oxygen is “electron greedy” Oxygen pulls electrons toward itself and away from hydrogen e- eee- O e- e- ee- H P+ P+ P+ H P+ This pulling of electrons toward itself is called “electronegativity”

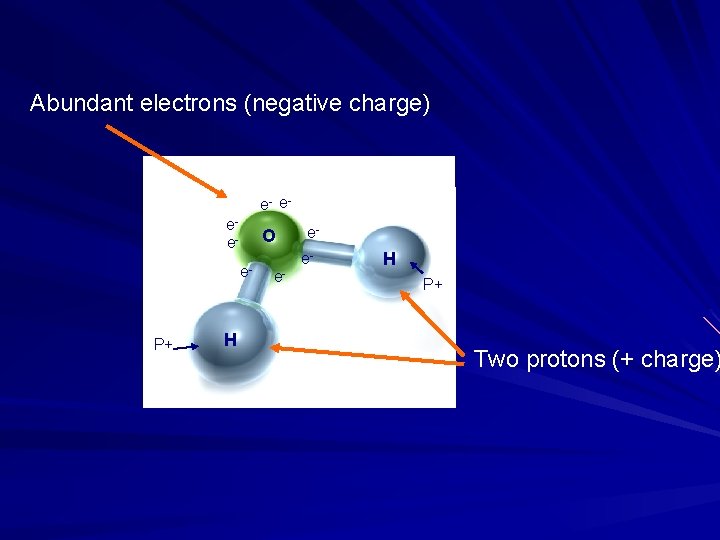
Abundant electrons (negative charge) e- eee- O e- P+ H e- ee- H P+ Two protons (+ charge)

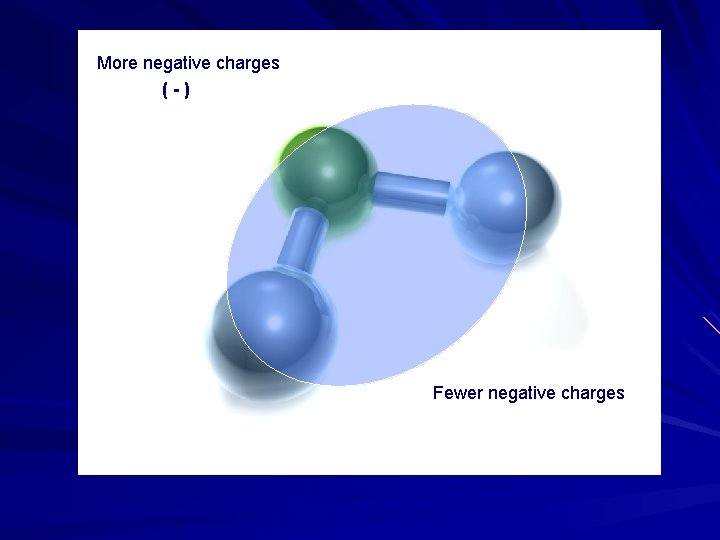
More negative charges (-) Fewer negative charges

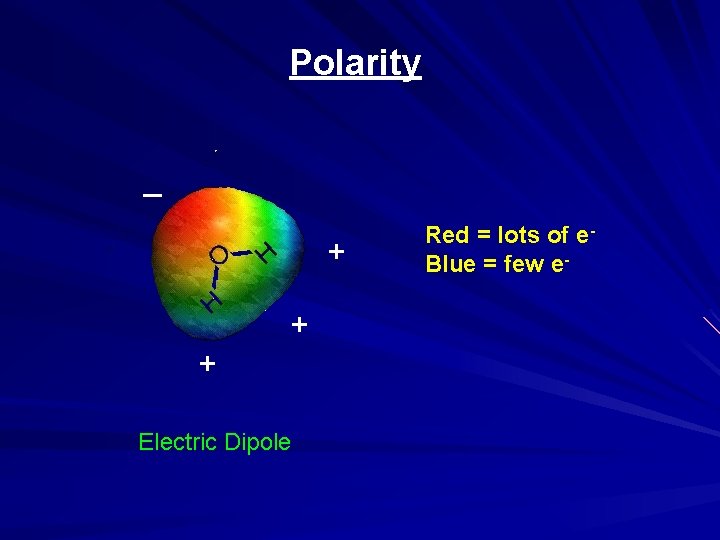
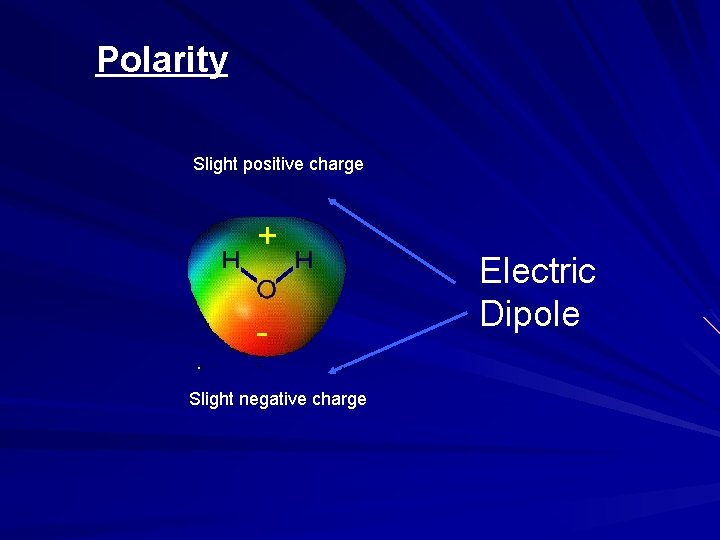
Polarity _ + + + Electric Dipole Red = lots of e. Blue = few e-

Consequences of Polarity

Magnets and Polarity N Magnetic Dipole S

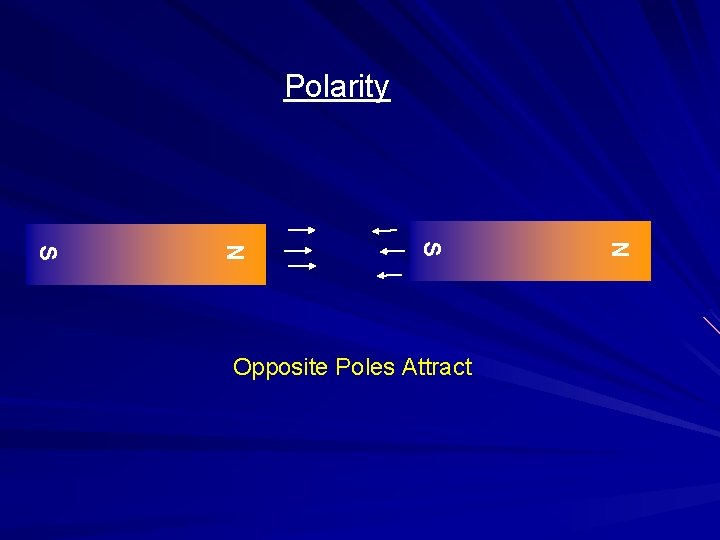
Polarity N S Opposite Poles Attract

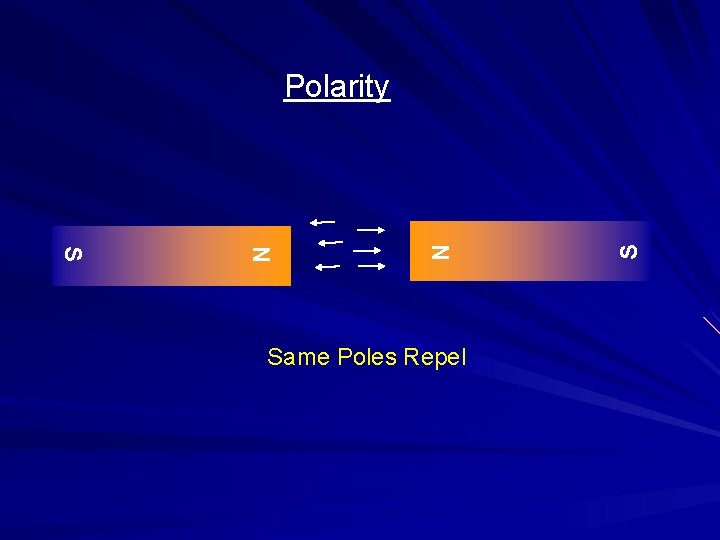
Same Poles Repel S N Polarity

Polarity N N S S http: //games. mochiads. com/c/g/polarityfreak-11 -Mochi-Sec. swf

Polarity + Slight positive charge Slight negative charge Electric Dipole

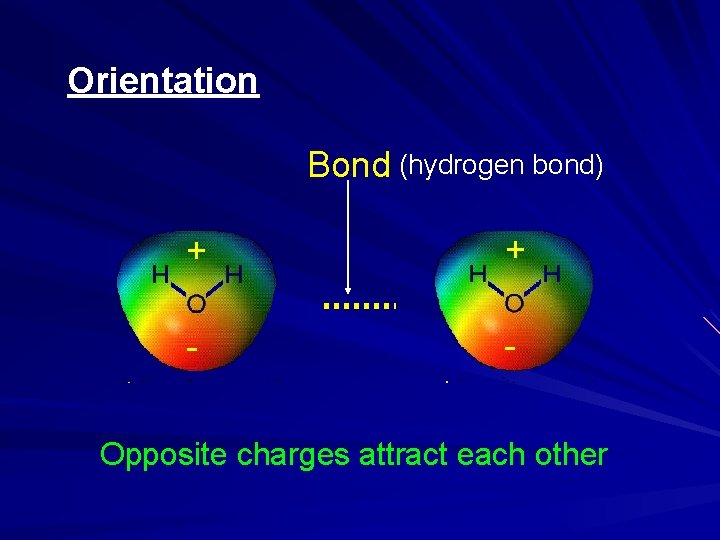
Orientation + + Bond (hydrogen bond) - - Opposite charges attract each other

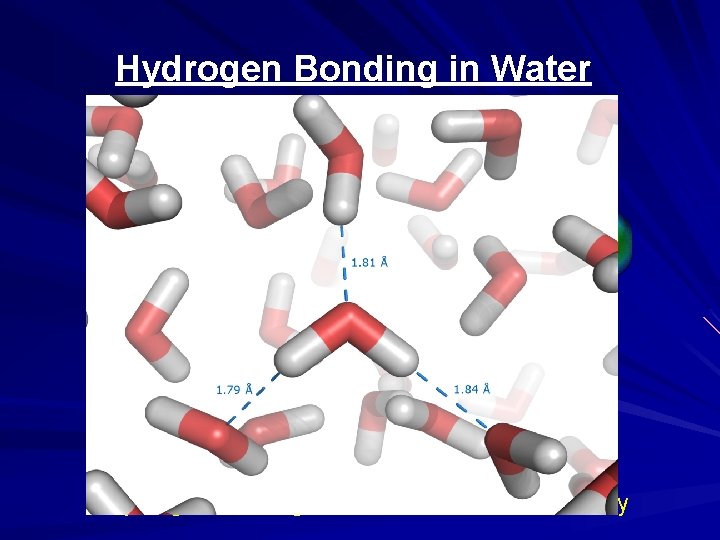
Hydrogen Bonding in Water Hydrogen Bonding Gives Water Unusual Stability

Summary Hydrogen and oxygen share electrons to form water The water molecule is electrically unbalanced Oxygen is electron greedy; it is highly electronegative Oxygen draws electrons toward itself and away from hydrogen This creates a slight negative charge near oxygen There is also a slight positive charge near hydrogen The result is a molecule that is polar (+ and – poles) This polarity accounts for electrostatic bonding between water molecules Bonding between water molecules gives water unusual stability.

Effect on Properties Extensive Hydrogen Bonding Allows Water to Exist as a Liquid at Normal Temperatures and across a wide range in temperatures High Boiling and Freezing Points Other Unusual Thermal Properties Unusual Density
 Hey hey bye bye
Hey hey bye bye Water and water and water water
Water and water and water water Dont ask why why why
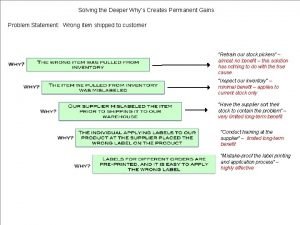
Dont ask why why why Why-why analysis
Why-why analysis Why do you cry, willie
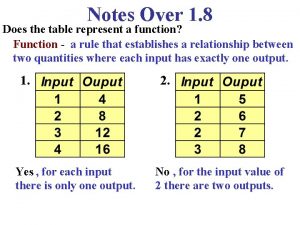
Why do you cry, willie Does the table represent a function why or why not
Does the table represent a function why or why not Does the table represent a function why or why not
Does the table represent a function why or why not Why or why not
Why or why not Contoh laporan root cause analysis
Contoh laporan root cause analysis Why does a star's life expectancy depend on mass
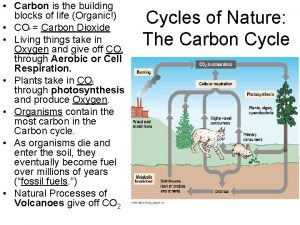
Why does a star's life expectancy depend on mass Why is carbon the building block of life
Why is carbon the building block of life Why did hason raja leave the life of comfort and pleasure
Why did hason raja leave the life of comfort and pleasure Threshold of life
Threshold of life Why was life on the manor often harsh for peasants
Why was life on the manor often harsh for peasants Lessons from a pencil
Lessons from a pencil Country life vocabulary
Country life vocabulary Farm life vs city life
Farm life vs city life Daily life real life polynomial problems
Daily life real life polynomial problems Single life and married life
Single life and married life Life orientation skills
Life orientation skills Country life vs city life compare /contrast
Country life vs city life compare /contrast City life vs country life
City life vs country life Moral lesson of life of pi
Moral lesson of life of pi Boundaries meme
Boundaries meme The life that is truly life
The life that is truly life John needham experiment main idea
John needham experiment main idea Freetutorical.com harvest land
Freetutorical.com harvest land Conclusion on mesopotamian civilization
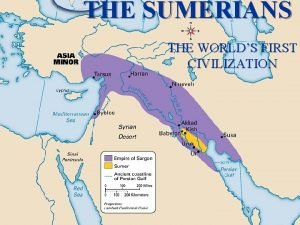
Conclusion on mesopotamian civilization Where were the sumerians
Where were the sumerians Where is aztec located
Where is aztec located Culture by edward taylor
Culture by edward taylor Ancient sumerians
Ancient sumerians