Water Chapter 3 Water n n n Life












![Fig. 3 -UN 5 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–] Basic Fig. 3 -UN 5 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–] Basic](https://slidetodoc.com/presentation_image_h2/5ea7c1a686bb5acfdfd0245f726b08f1/image-20.jpg)



- Slides: 27

Water Chapter 3

Water n n n Life began in water 2/3’s of an organisms body Organisms grow or reproduce in a water-rich environment.

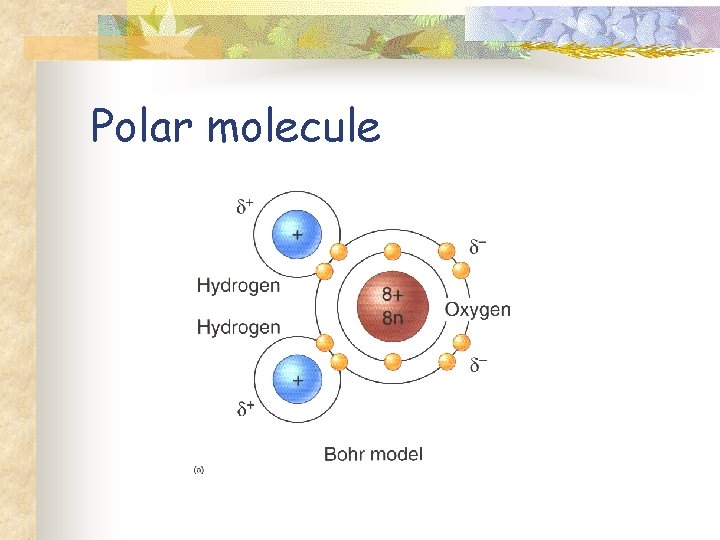
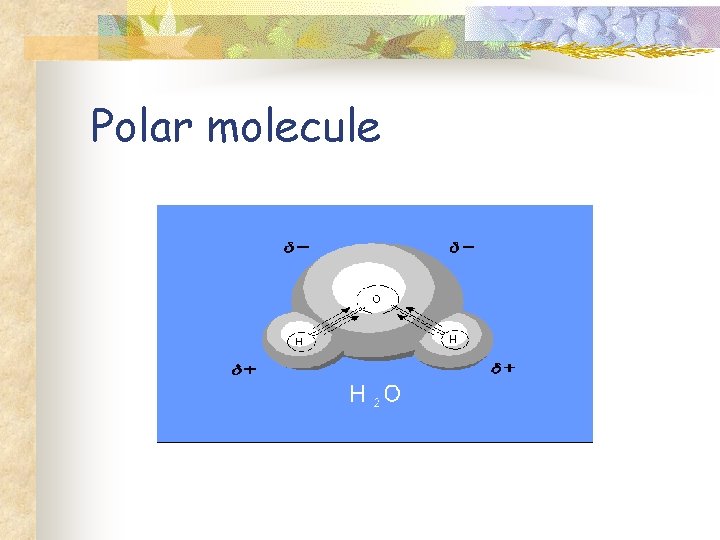
Water Molecule n n n Covalent bonding Oxygen is more electronegative Polar molecule Polarity of water underlies its chemistry Chemistry of life.

Polar molecule

Polar molecule

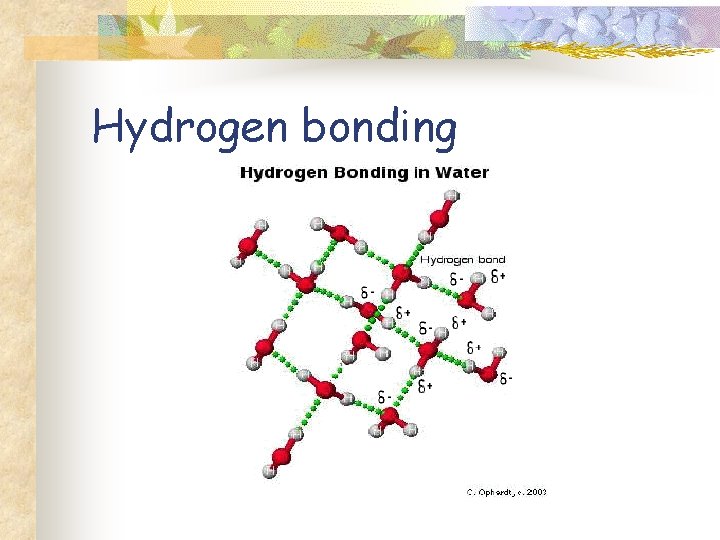
Hydrogen bonding


Properties of water • • • Cohesion: Attraction between water molecules Responsible for surface tension of water

Properties of water n n n Adhesion: Attraction of water between other molecules Capillary action

Properties of water n n n Moderation of water temperature Water is a liquid at moderate temperatures Specific heat: Amount of heat needed to a raise 1 gram of a substance 10 Celsius Water’s specific heat is 1 calorie/gram/0 C (4. 18 Joules)

Properties of water n n Evaporative cooling Heat of vaporization: Amount of heat needed to change 1 gram of a substance from a liquid to a gas. 586 Calories (2260 Joules)

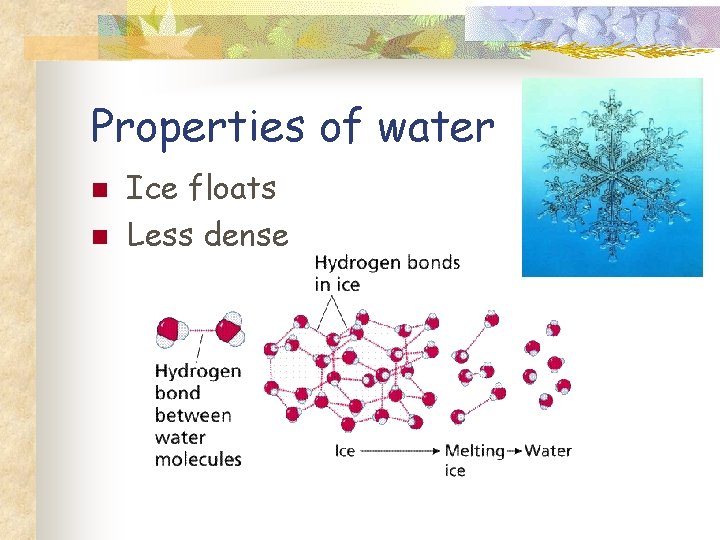
Properties of water n n Ice floats Less dense

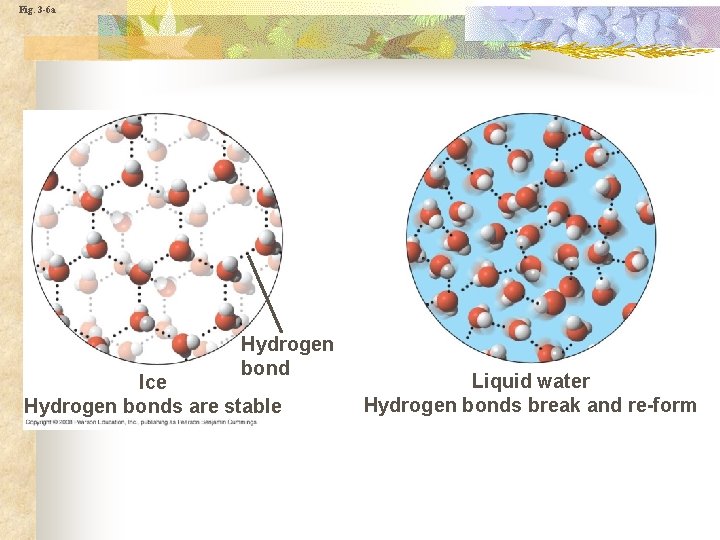
Fig. 3 -6 a Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form

Properties of water n n n Solvent Water surrounds ionic & polar molecules Table salt or sugar Aqueous solution Molarity


Hydrophobic n n “fear” of water Common in non-polar molecules Non-polar molecules tend to aggregate in water Hydrophobic exclusion

Hydrophilic n n “water-loving” Common in polar molecules

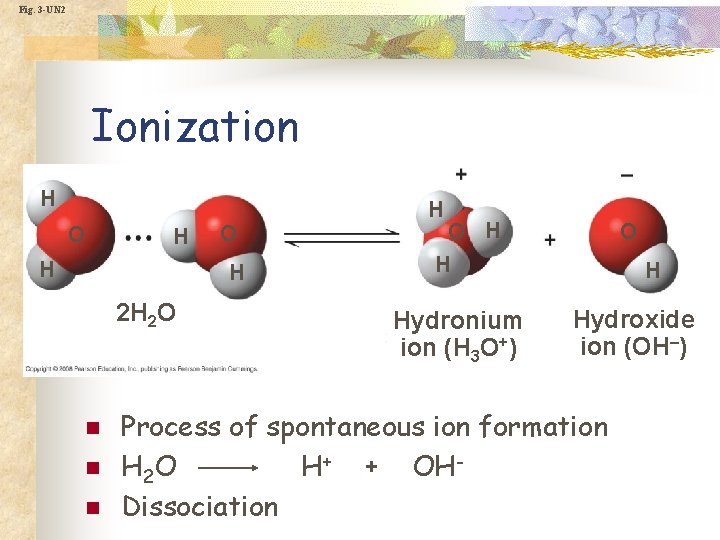
Fig. 3 -UN 2 Ionization H O H 2 H 2 O n n n H O H H Hydronium ion (H 3 O+) O H Hydroxide ion (OH–) Process of spontaneous ion formation H 2 O H+ + OHDissociation

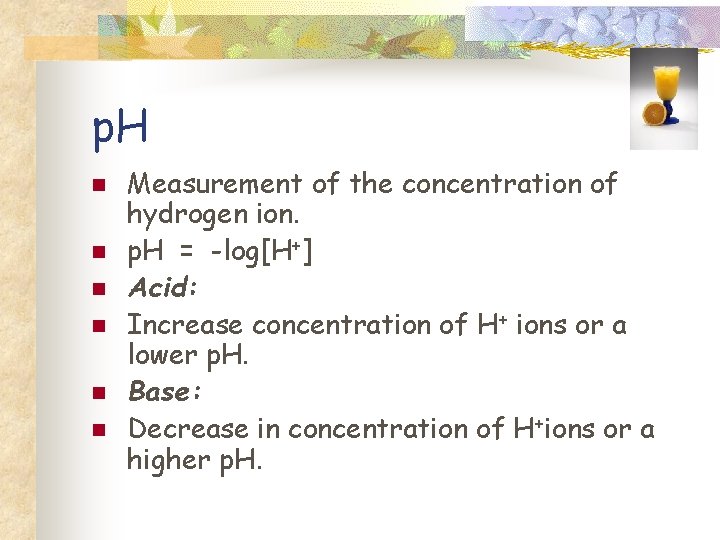
p. H n n n Measurement of the concentration of hydrogen ion. p. H = -log[H+] Acid: Increase concentration of H+ ions or a lower p. H. Base: Decrease in concentration of H+ions or a higher p. H.
![Fig 3 UN 5 0 Acidic H OH Neutral H OH Basic Fig. 3 -UN 5 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–] Basic](https://slidetodoc.com/presentation_image_h2/5ea7c1a686bb5acfdfd0245f726b08f1/image-20.jpg)
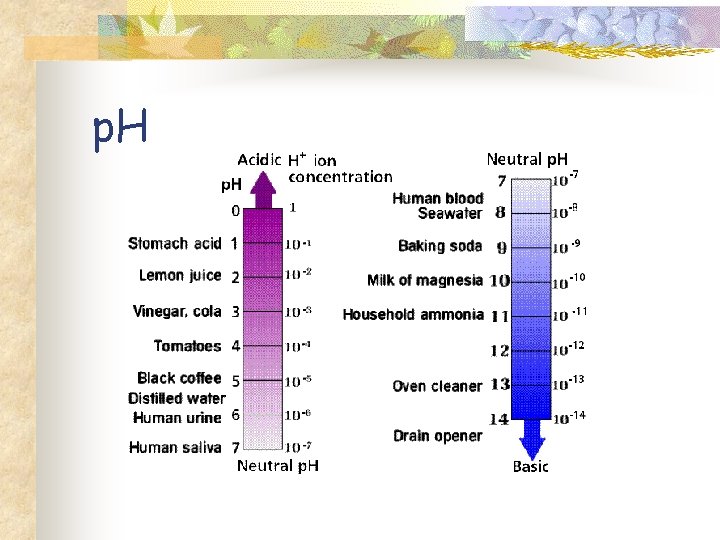
Fig. 3 -UN 5 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–] Basic [H+] < [OH–] Acids donate H+ in aqueous solutions 7 Bases donate OH– or accept H+ in aqueous solutions 14

p. H

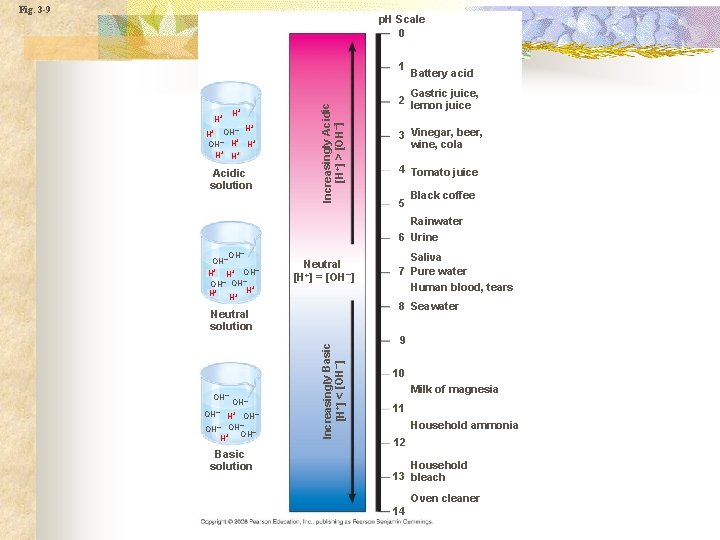
Fig. 3 -9 p. H Scale 0 H+ H+ + – H H+ OH– H H+ H+ H+ Acidic solution Increasingly Acidic [H+] > [OH–] 1 Battery acid Gastric juice, 2 lemon juice 3 Vinegar, beer, wine, cola 4 Tomato juice 5 Black coffee Rainwater 6 Urine OH– H+ OH– OH– + H+ H+ H Neutral [H+] = [OH–] 8 Seawater OH– H+ OH– – OH OH– + H Basic solution Increasingly Basic [H+] < [OH–] Neutral solution OH– Saliva 7 Pure water Human blood, tears 9 10 Milk of magnesia 11 Household ammonia 12 Household 13 bleach Oven cleaner 14

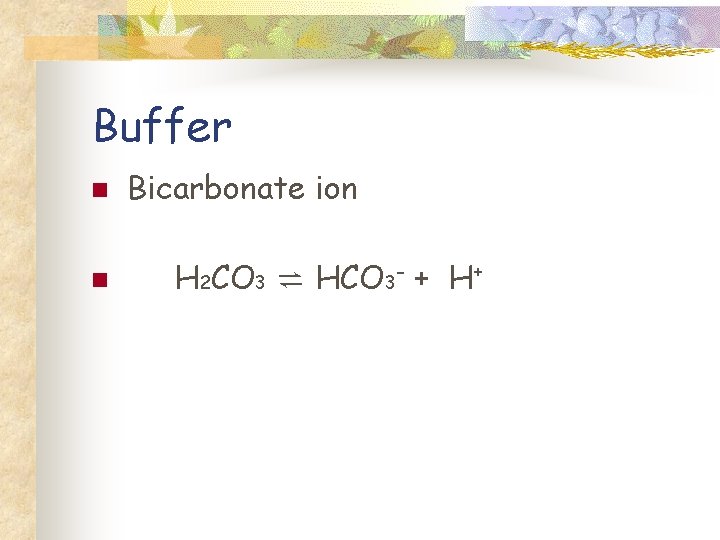
Buffer n n n Substance helps maintain a balanced p. H Accepts H+ ions when excess Donates when there are too few.

Buffer n n Blood p. H is approximately 7. 4. Bicarbonate ion helps maintain the p. H of the blood. Blood acidosis: p. H drops 0. 2 to 0. 4 points on the p. H scale Blood alkalosis: p. H goes up 0. 2 to 0. 4 points on the p. H scale

Buffer n n Bicarbonate ion H 2 CO 3 ⇌ HCO 3 - + H+

Acid rain n n Sulfuric acid Nitric acid

Acid rain
 Water and water and water water
Water and water and water water Nekton include all animals that
Nekton include all animals that Water and life chapter 3
Water and life chapter 3 Covalent bonding in water
Covalent bonding in water A paved blacktop parking lot was built
A paved blacktop parking lot was built Chapter 9 surface water answer key
Chapter 9 surface water answer key City life vocabulary
City life vocabulary Farm life vs city life
Farm life vs city life Adding polynomials examples
Adding polynomials examples Single life vs married life quotes
Single life vs married life quotes Slidetodoc.com
Slidetodoc.com Country life vs city life compare /contrast
Country life vs city life compare /contrast City life vs country life
City life vs country life Lessons from life of pi
Lessons from life of pi How do we treat the life the life how we treat
How do we treat the life the life how we treat Life that is truly life
Life that is truly life The idea life comes from life is
The idea life comes from life is Unit 8 country life and city life
Unit 8 country life and city life Water an elixir of life essay
Water an elixir of life essay Enagic founder
Enagic founder Importance of water in human life
Importance of water in human life Lifewatermedia
Lifewatermedia Humankind has always searched in vain
Humankind has always searched in vain What are the life supporting properties of water
What are the life supporting properties of water Water consumption water meter reading worksheet
Water consumption water meter reading worksheet Fwa kapal adalah
Fwa kapal adalah Water o water
Water o water Estimating products of fractions
Estimating products of fractions