Volumetric Analysis Oxidationreduction Ch 15 Potassium Permanganate Oxidising





- Slides: 17

Volumetric Analysis Oxidation-reduction, Ch 15

Potassium Permanganate: Oxidising Agent • KMn. O 4 • Potassium Manganate (VII) • Not a primary standard • Not available in highly pure state • Decomposes in sunlight • Oxidising agent – gains 5 electrons under acidic conditions

Potassium Permanganate: Oxidising Agent • If forget to add sulphuric acid, • Oxidation states: • +7: Pink/purple • +4: Brown • +2 Colourless • NB: must use sulphuric acid as • HCl contains Cl- which can be oxidised to Cl 2 • HNO 3 is an oxidising agent

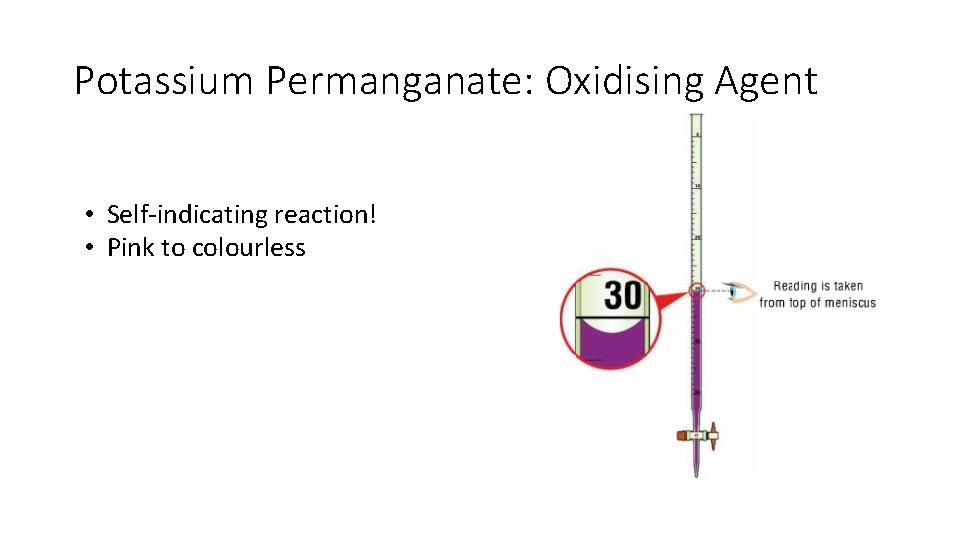
Potassium Permanganate: Oxidising Agent • Self-indicating reaction! • Pink to colourless

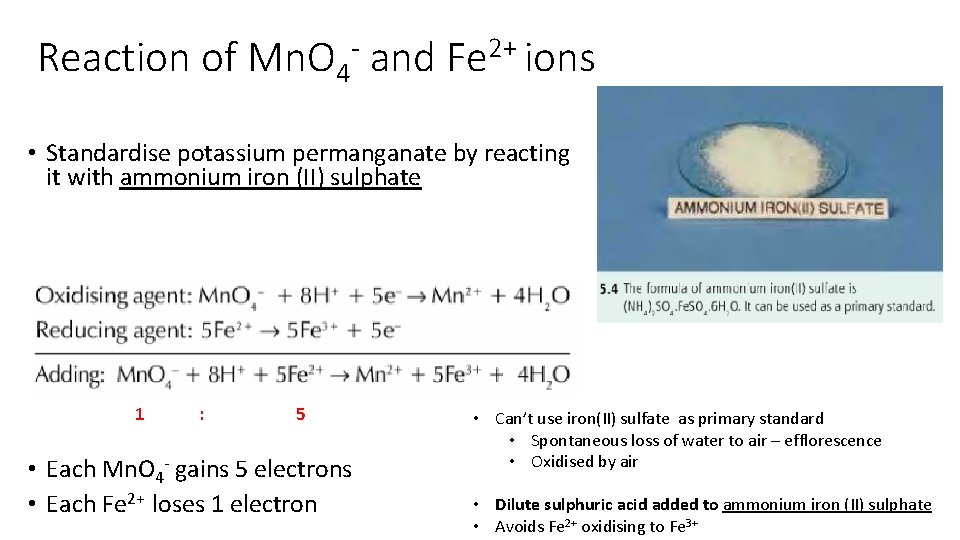
Reaction of Mn. O 4 - and Fe 2+ ions • Standardise potassium permanganate by reacting it with ammonium iron (II) sulphate 1 : 5 • Each Mn. O 4 - gains 5 electrons • Each Fe 2+ loses 1 electron • Can’t use iron(II) sulfate as primary standard • Spontaneous loss of water to air – efflorescence • Oxidised by air • Dilute sulphuric acid added to ammonium iron (II) sulphate • Avoids Fe 2+ oxidising to Fe 3+

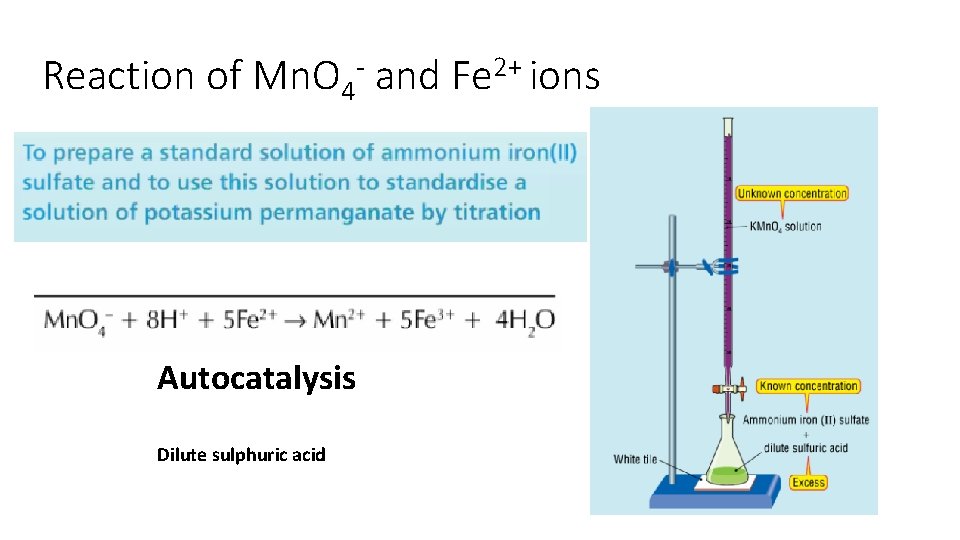
Reaction of Mn. O 4 - and Fe 2+ ions Autocatalysis Dilute sulphuric acid

Determine amount of iron in an iron tablet • Iron supplement taken to treat/prevent anaemia • Iron needed in haemoglobin • Iron tablets contain anhydrous iron(II) sulphate • Fe. SO 4 • Titrate iron tablet solution against solution of potassium permanganate (known concentration)

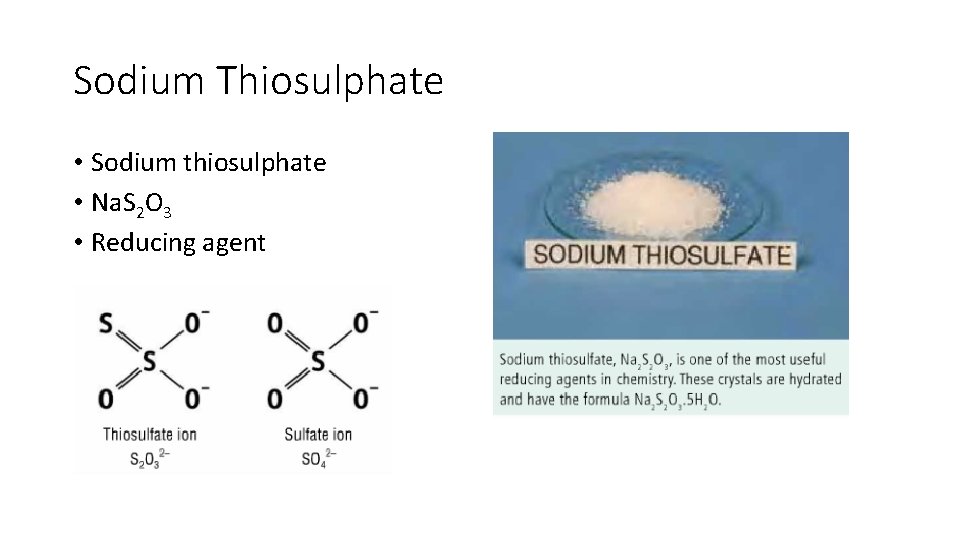
Sodium Thiosulphate • Sodium thiosulphate • Na. S 2 O 3 • Reducing agent

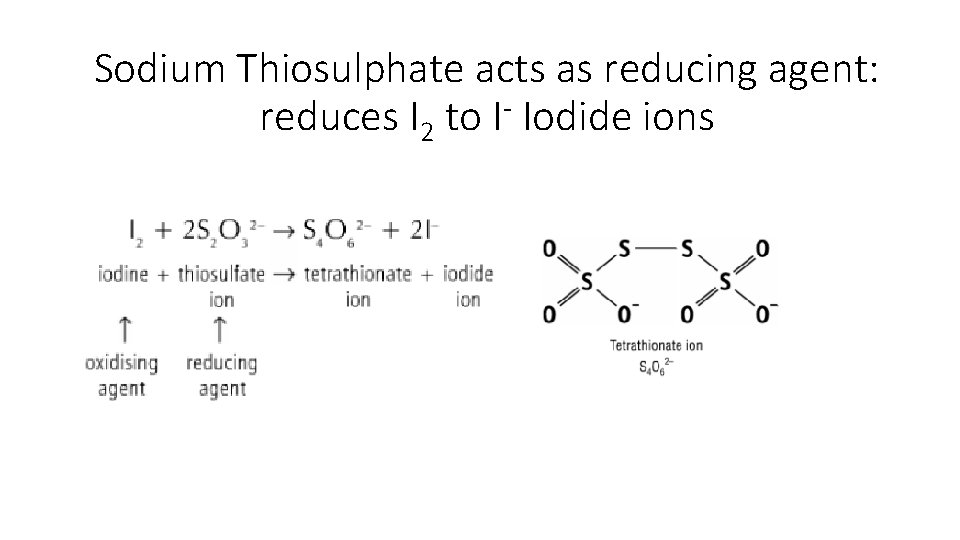
Sodium Thiosulphate acts as reducing agent: reduces I 2 to I- Iodide ions

Sodium Thiosulphate • Not a primary standard • Not available in sufficiently pure state • Efflorescent • Standardised by titrating against standard solution of iodine

Iodine • Standard solution of iodine can’t be made by direct weighing • Iodine slightly vaporises/sublimes • Insoluble in water • Not a primary standard

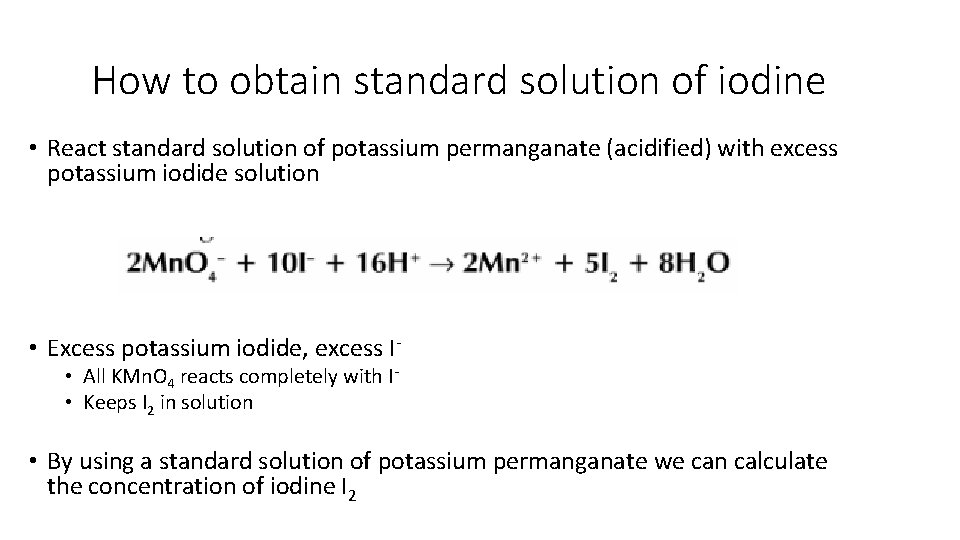
How to obtain standard solution of iodine • React standard solution of potassium permanganate (acidified) with excess potassium iodide solution • Excess potassium iodide, excess I • All KMn. O 4 reacts completely with I • Keeps I 2 in solution • By using a standard solution of potassium permanganate we can calculate the concentration of iodine I 2

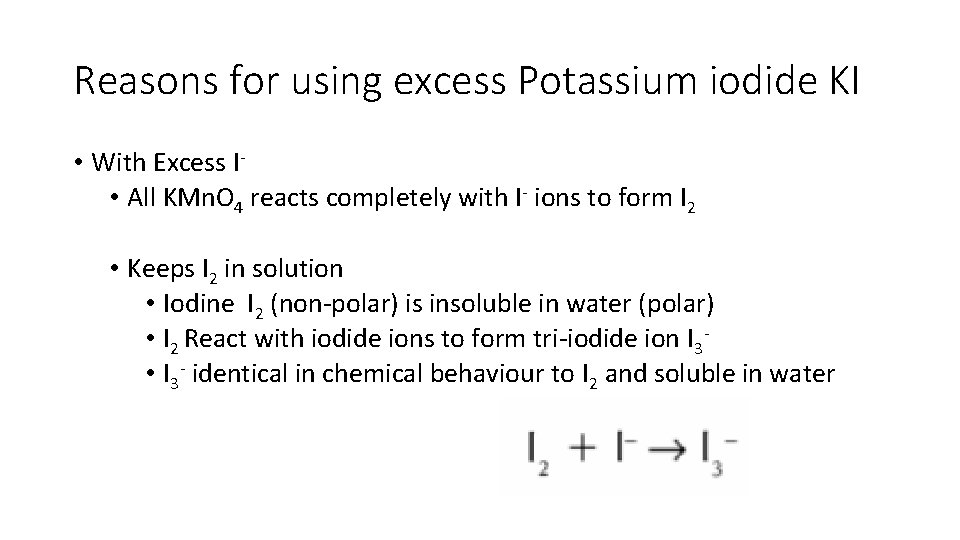
Reasons for using excess Potassium iodide KI • With Excess I • All KMn. O 4 reacts completely with I- ions to form I 2 • Keeps I 2 in solution • Iodine I 2 (non-polar) is insoluble in water (polar) • I 2 React with iodide ions to form tri-iodide ion I 3 • I 3 - identical in chemical behaviour to I 2 and soluble in water


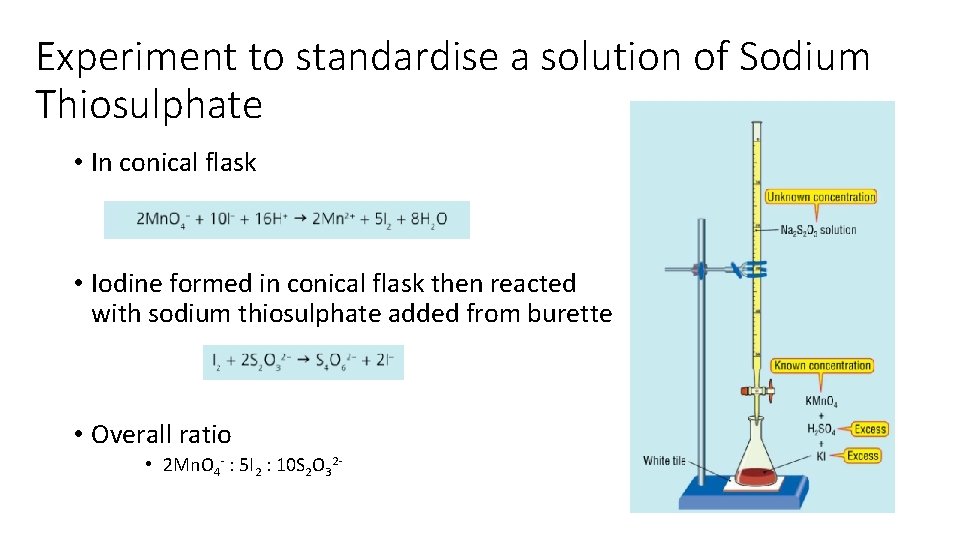
Experiment to standardise a solution of Sodium Thiosulphate • In conical flask • Iodine formed in conical flask then reacted with sodium thiosulphate added from burette • Overall ratio • 2 Mn. O 4 - : 5 I 2 : 10 S 2 O 32 -

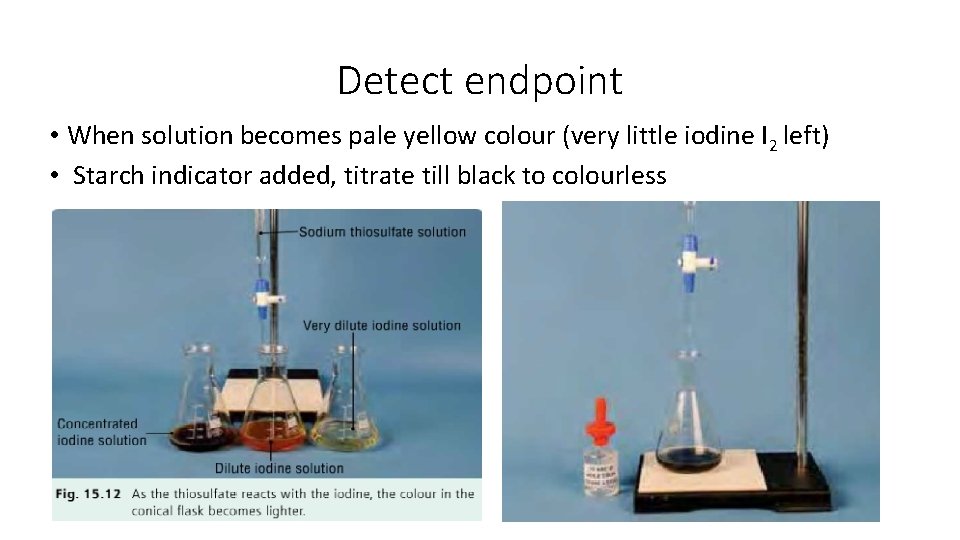
Detect endpoint • When solution becomes pale yellow colour (very little iodine I 2 left) • Starch indicator added, titrate till black to colourless

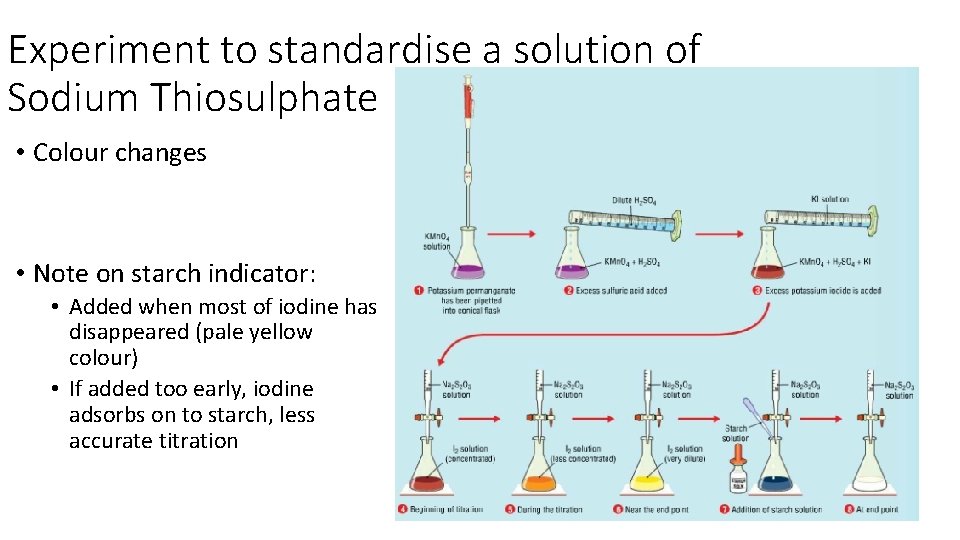
Experiment to standardise a solution of Sodium Thiosulphate • Colour changes • Note on starch indicator: • Added when most of iodine has disappeared (pale yellow colour) • If added too early, iodine adsorbs on to starch, less accurate titration