Volumetric analysis Chemical reaction in volumetric analysis o





- Slides: 11


Volumetric analysis Chemical reaction in volumetric analysis : o o Acid-base or (vice versa) titrations Precipitation titrations Complex – formation titrations Oxidation – reduction (redox) titrations

Titration Chemical analysis which can be used to determine the concentration of a known reactant called titrant (B) ( standard solution ) and volume is used to react with a measured quantity of reactant (Analyte) (A) that has been consumed when the Endpoint of titration is reached. Because volume measurements play a key role in titration , it is known as volumetric analysis. a. A+b. B r. R

Condition of titration reaction: q The reaction should be rapid , complete , and possible to describe by balanced chemical reaction and equal. q There must be a clear change in the nature of the solution at the endpoint of the reaction. q The appropriate indicator should be available to indicate the endpoint of the reaction.

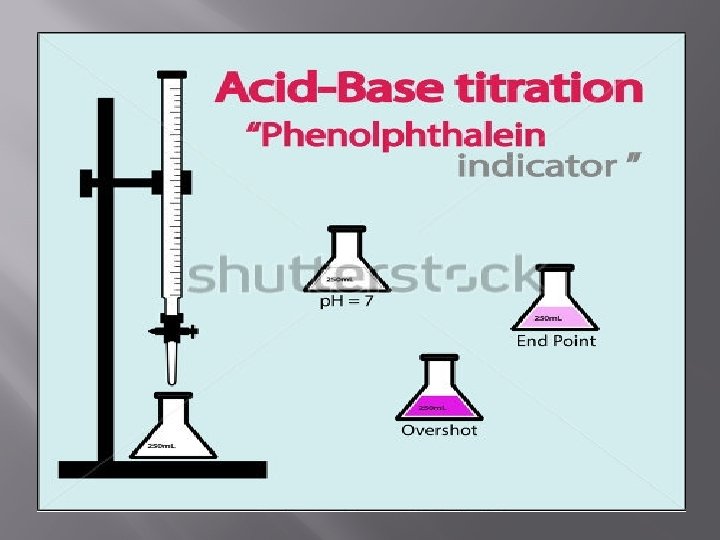
Acid-Base Titrations Are based on the neutralization reaction between the analyte and an acidic or basic titrant. Neutralization is also called a water formation reaction. • A strong acid react with a strong base to form a neutral (PH=7) solution. • A strong acid react with a weak base to form an acidic (PH<7) solution. • A weak acid react with a strong base to form an a basic (PH>7) solution. • Na. OH + HCL Na. CL+ H 2 O

Standard solution Is a solution containing the specified volume of a known weight of the dissolved substance. Characteristics of standard material : 1. Be of high purity. 2. With known chemical composition. 3. Stable 4. With high equivalent weight. 5. Easy solubility in water and proper solvents.

Indicator It is a weak acid or base and there is a combination of one electrolyte and the other is not electrolyte and are in equilibrium with some. HIn colorless not electrolyte balanc e In. H dissociation H+ + In- electrolyte weak red pink End point : The point at which the reaction ends where a clear change in the color of the solution occurs.


Standardization of Na. OH A. B. C. D. E. F. G. H. Fill the burette with an HCL solution of known concentration Drag 10 ml of Na. OH solution of know concentration 0. 1 N into a conical flask Add 2 drops from phenolphtalin indicator. Titrate Na. OH solution by adding HCL from the burette slowly with constant stirring until the color turns from pink red to colorless. Calculate the volume down of HCL from the burette. Repeat the previous steps again so that you reach a volume close to the first volume difference between them is very little ±( 0. 1 -0. 3 ). Calculate the basicity of Na. OH of the following relationship N 1 V 1= N 2 V 2 Calculate the concentration of Na. OH per liter of solution.

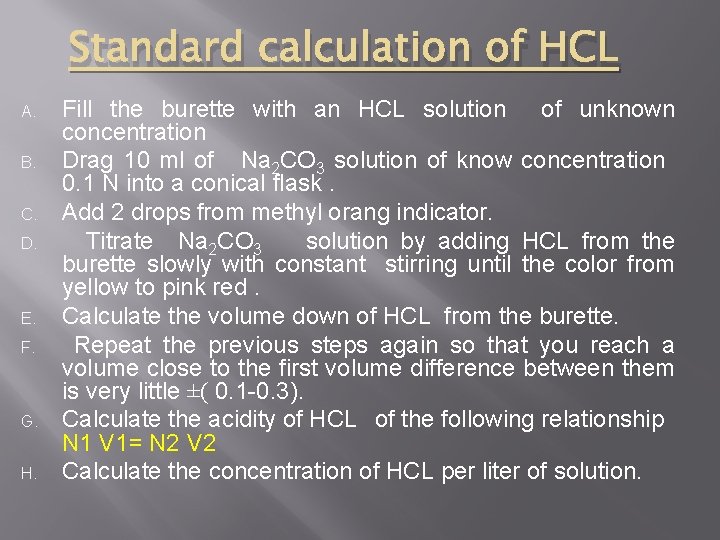
Standard calculation of HCL A. B. C. D. E. F. G. H. Fill the burette with an HCL solution of unknown concentration Drag 10 ml of Na 2 CO 3 solution of know concentration 0. 1 N into a conical flask. Add 2 drops from methyl orang indicator. Titrate Na 2 CO 3 solution by adding HCL from the burette slowly with constant stirring until the color from yellow to pink red. Calculate the volume down of HCL from the burette. Repeat the previous steps again so that you reach a volume close to the first volume difference between them is very little ±( 0. 1 -0. 3). Calculate the acidity of HCL of the following relationship N 1 V 1= N 2 V 2 Calculate the concentration of HCL per liter of solution.

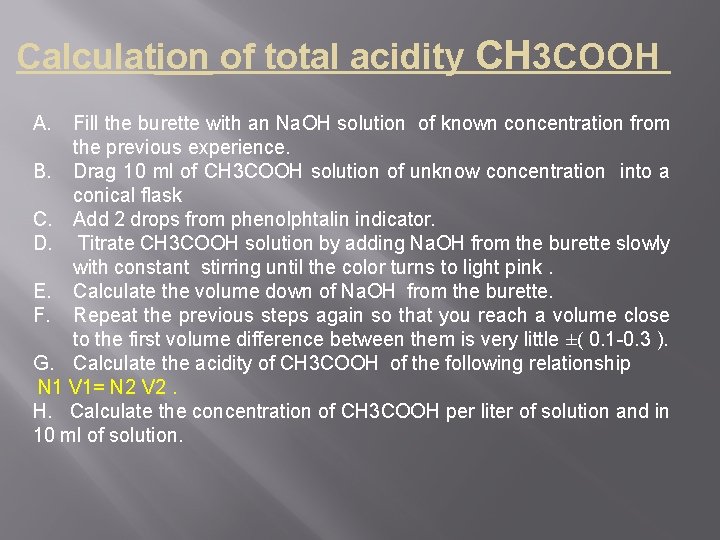
Calculation of total acidity CH 3 COOH A. Fill the burette with an Na. OH solution of known concentration from the previous experience. B. Drag 10 ml of CH 3 COOH solution of unknow concentration into a conical flask C. Add 2 drops from phenolphtalin indicator. D. Titrate CH 3 COOH solution by adding Na. OH from the burette slowly with constant stirring until the color turns to light pink. E. Calculate the volume down of Na. OH from the burette. F. Repeat the previous steps again so that you reach a volume close to the first volume difference between them is very little ±( 0. 1 -0. 3 ). G. Calculate the acidity of CH 3 COOH of the following relationship N 1 V 1= N 2 V 2. H. Calculate the concentration of CH 3 COOH per liter of solution and in 10 ml of solution.