Presentation Topic Potassium permanganate and Potassium dichromate is







- Slides: 11

Presentation Topic “Potassium permanganate and Potassium dichromate is an excellent tool in analysis” www. Assignment. Point. com

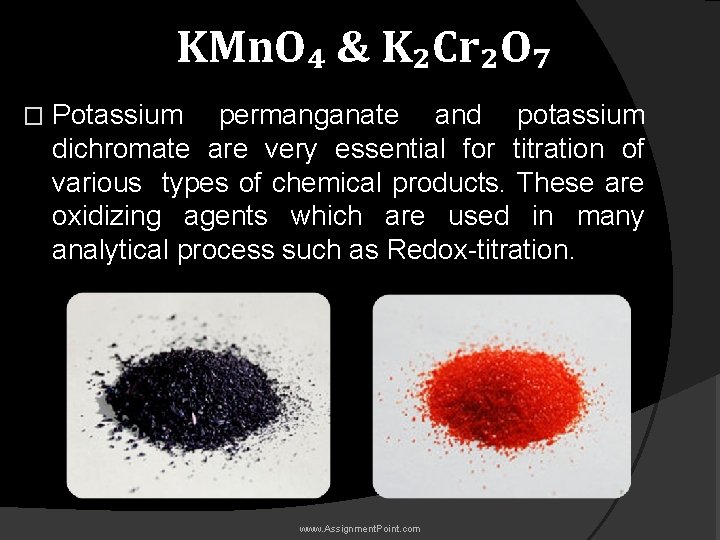
KMn. O₄ & K₂Cr₂O₇ � Potassium permanganate and potassium dichromate are very essential for titration of various types of chemical products. These are oxidizing agents which are used in many analytical process such as Redox-titration. www. Assignment. Point. com

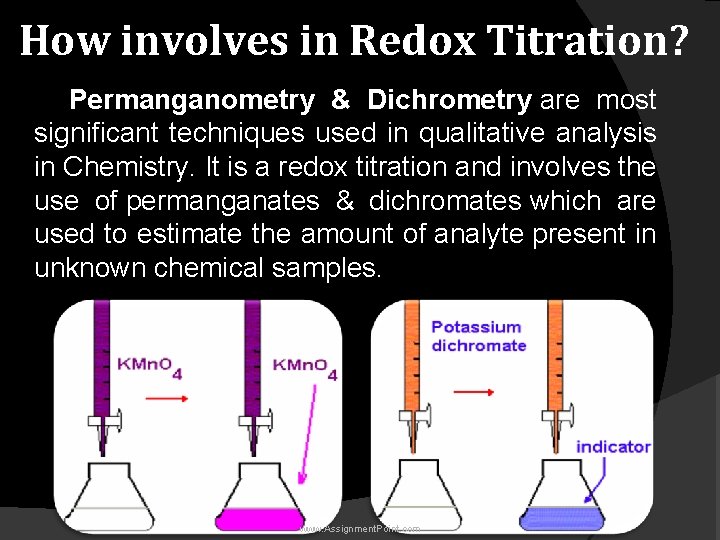
How involves in Redox Titration? Permanganometry & Dichrometry are most significant techniques used in qualitative analysis in Chemistry. It is a redox titration and involves the use of permanganates & dichromates which are used to estimate the amount of analyte present in unknown chemical samples. www. Assignment. Point. com

Justification between KMn. O₄ & K₂Cr₂O₇ : � KMn. O₄: The major application of KMn. O 4 is as a reagent for the synthesis of organic compounds. Significant amounts are required for the synthesis of ascorbic acid, chloramphenicol, saccharin, isonicotinic acid, and pyrazinoic acid. � K₂Cr₂O₇: It can be used to test for sulfur dioxide, as it turns distinctively from orange to green. www. Assignment. Point. com

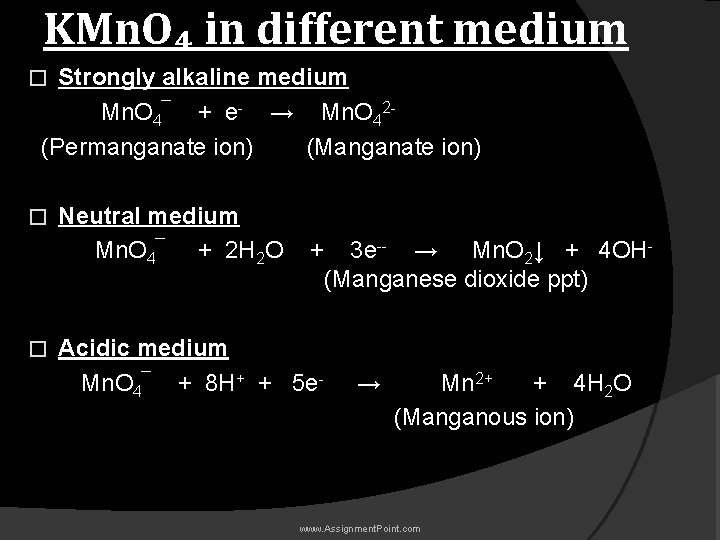
KMn. O₄ in different medium Strongly alkaline medium Mn. O 4¯ + e- → Mn. O 42(Permanganate ion) (Manganate ion) � � � Neutral medium Mn. O 4¯ + 2 H 2 O + 3 e-- → Mn. O 2↓ + 4 OH(Manganese dioxide ppt) Acidic medium Mn. O 4¯ + 8 H+ + 5 e- → Mn 2+ + 4 H 2 O (Manganous ion) www. Assignment. Point. com

K₂Cr₂O₇ in Acidic medium: � Potassium dichromate acts as oxidizing agent in acidic medium only. Cr 2 O 72– + 14 H+ + 6 e– → 2 Cr 3+ + 7 H 2 O www. Assignment. Point. com

Justification � KMn. O₄ is not a secondary standard. It is not easily obtained in perfectly pure form which is completely free from manganese dioxide. � K₂Cr₂O₇ is an excellent primary standard and available in high purity. Its aqueous solutions are not attacked by oxidisable impurities like rubber or any other organic matter. www. Assignment. Point. com

Advantages of KMn. O₄ & K₂Cr₂O₇ Advantage of KMn. O₄: Advantage of K₂Cr₂O₇: Low cost. Having specific color. Act as self indicator. Titration can be perform in both acidic and basic media. Easily can be prepared. Primary standard solution Instantly can use. Light can not do any harm. www. Assignment. Point. com

Why this two compound considered as an excellent tool in analysis? Potassium permanganate and potassium dichromate are widely using almost every sectors of analysis. As they are oxidizing agent they are greatly using in different types of titration. Safety handling and proper use of these reagent can ensure a perfect result. www. Assignment. Point. com

So we can say that. . www. Assignment. Point. com

Thank You All www. Assignment. Point. com