Principles of Volumetric Analysis titration titrant analyte indicator



![Precipitation Titration Curve p-function p. X = - log 10[X] precipitation titration curve four Precipitation Titration Curve p-function p. X = - log 10[X] precipitation titration curve four](https://slidetodoc.com/presentation_image_h/a3004c7ac66ca6d6523914af0719e5fa/image-11.jpg)

- Slides: 44

Principles of Volumetric Analysis titration titrant analyte indicator equivalence point vs. end point titration error blank titration Dr. S. M. Condren

Principles of Volumetric Analysis primary standard 1. High purity 100. 02% 2. Stability toward air 3. Absence of hydrate water 4. Available at moderate cost 5. Soluble Dr. S. M. Condren

Principles of Volumetric Analysis standardization standard solution Methods for establishing concentration direct method standardization secondary standard solution Dr. S. M. Condren

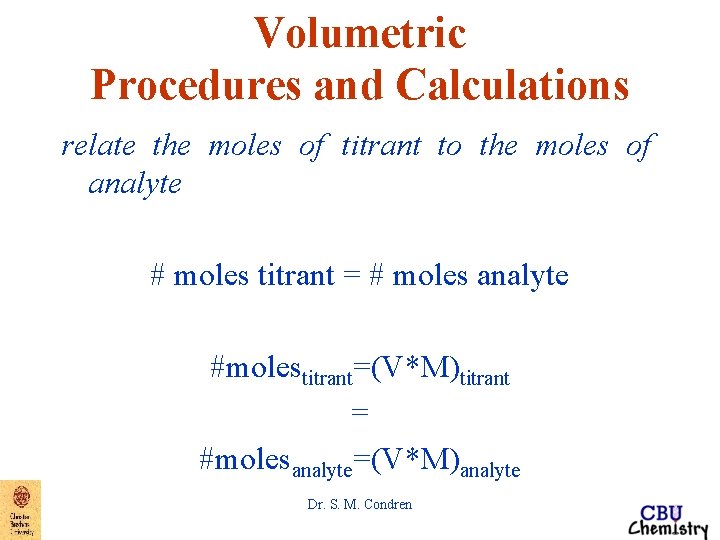
Volumetric Procedures and Calculations relate the moles of titrant to the moles of analyte # moles titrant = # moles analyte #molestitrant=(V*M)titrant = #molesanalyte=(V*M)analyte Dr. S. M. Condren

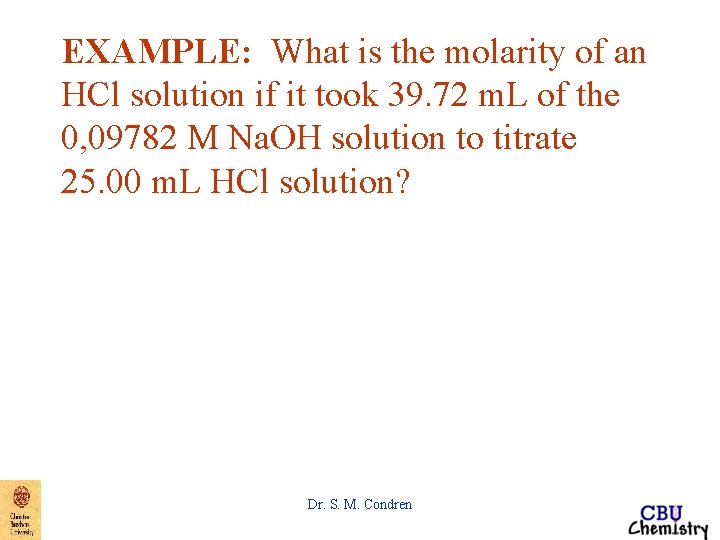
EXAMPLE: What is the molarity of an HCl solution if it took 39. 72 m. L of the 0, 09782 M Na. OH solution to titrate 25. 00 m. L HCl solution? Dr. S. M. Condren

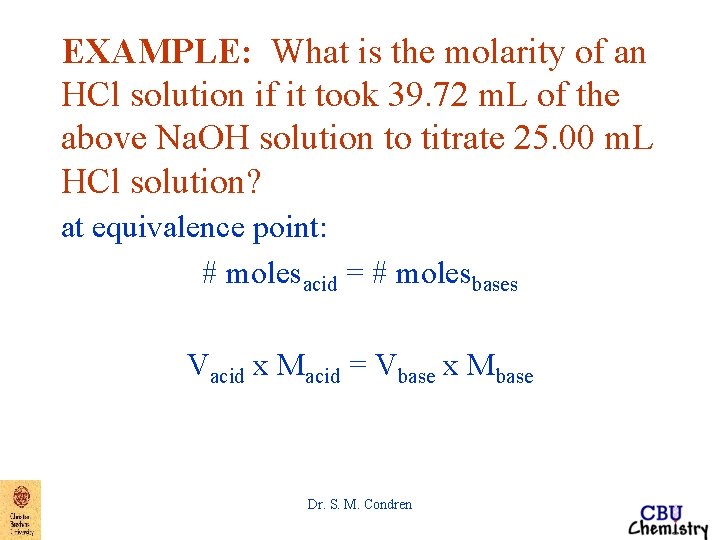
EXAMPLE: What is the molarity of an HCl solution if it took 39. 72 m. L of the above Na. OH solution to titrate 25. 00 m. L HCl solution? at equivalence point: # molesacid = # molesbases Vacid x Macid = Vbase x Mbase Dr. S. M. Condren

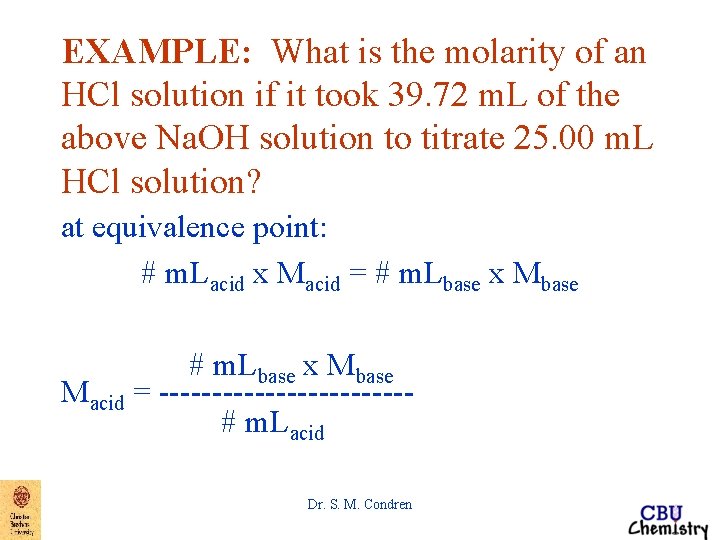
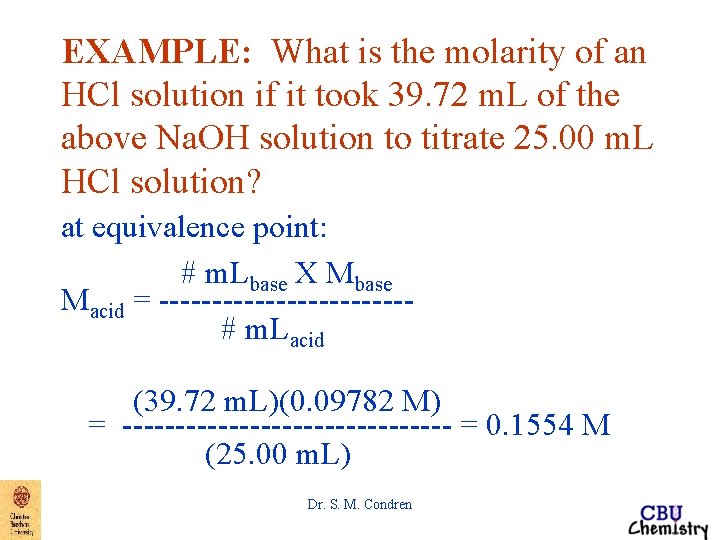
EXAMPLE: What is the molarity of an HCl solution if it took 39. 72 m. L of the above Na. OH solution to titrate 25. 00 m. L HCl solution? at equivalence point: # m. Lacid x Macid = # m. Lbase x Mbase Macid = ------------# m. Lacid Dr. S. M. Condren

EXAMPLE: What is the molarity of an HCl solution if it took 39. 72 m. L of the above Na. OH solution to titrate 25. 00 m. L HCl solution? at equivalence point: # m. Lbase X Mbase Macid = ------------# m. Lacid (39. 72 m. L)(0. 09782 M) = ---------------- = 0. 1554 M (25. 00 m. L) Dr. S. M. Condren

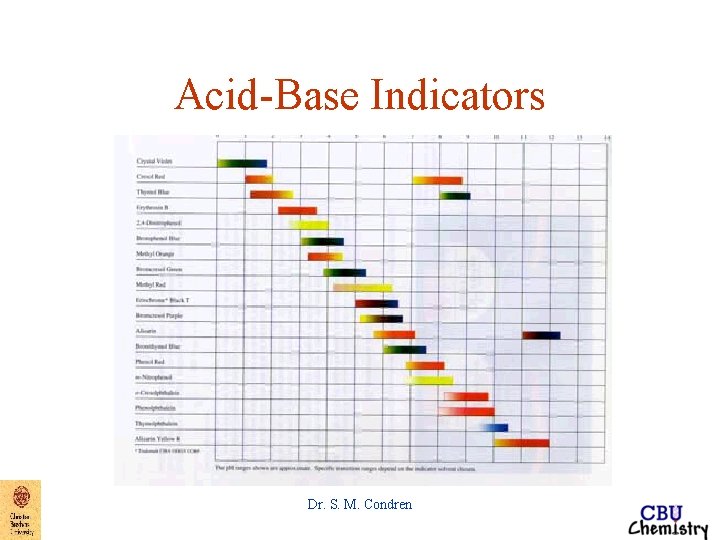
Acid-Base Indicators Dr. S. M. Condren

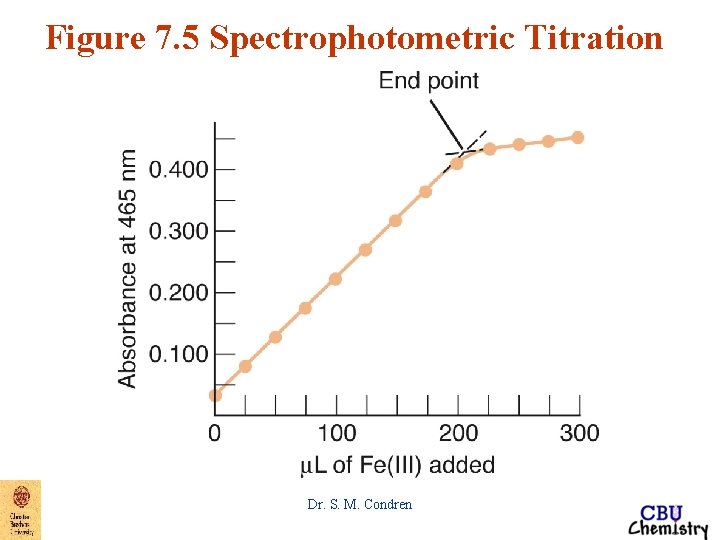
Figure 7. 5 Spectrophotometric Titration Dr. S. M. Condren
![Precipitation Titration Curve pfunction p X log 10X precipitation titration curve four Precipitation Titration Curve p-function p. X = - log 10[X] precipitation titration curve four](https://slidetodoc.com/presentation_image_h/a3004c7ac66ca6d6523914af0719e5fa/image-11.jpg)
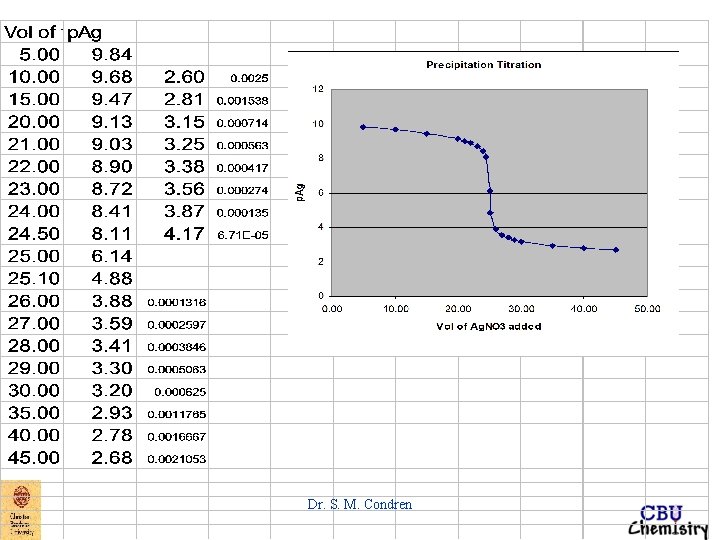
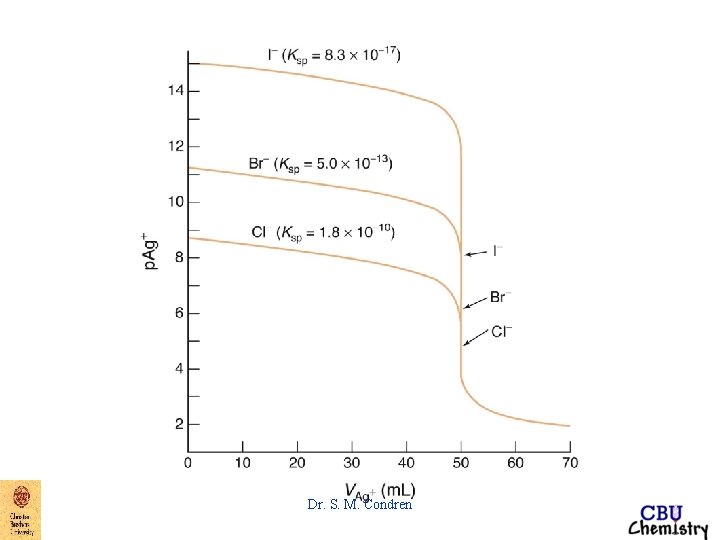
Precipitation Titration Curve p-function p. X = - log 10[X] precipitation titration curve four types of calculations initial point before equivalence point after equivalence point Dr. S. M. Condren

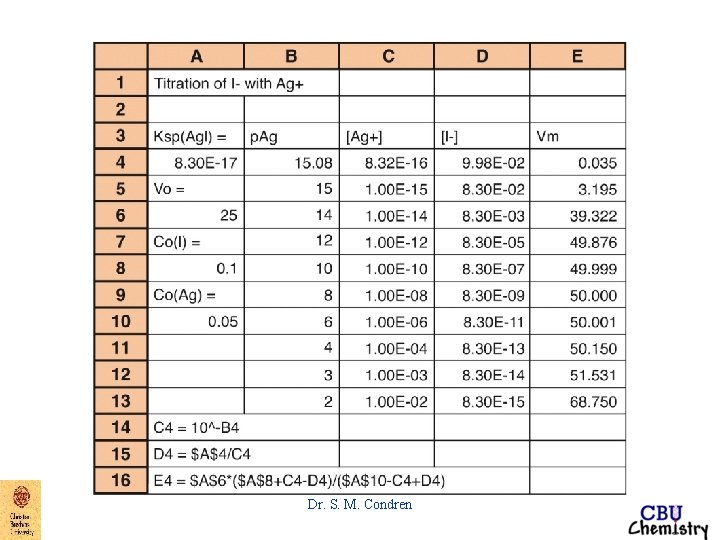
Precipitation Titration Curve EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. titration curve => p. Ag vs. vol. Ag. NO 3 added Dr. S. M. Condren

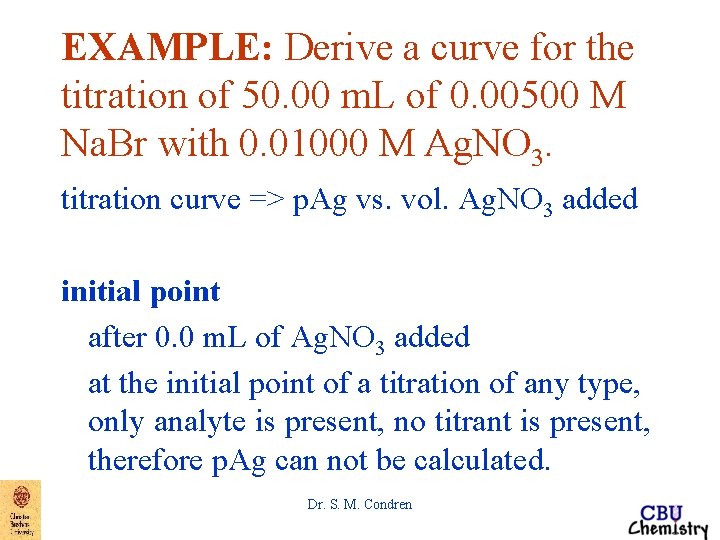
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. titration curve => p. Ag vs. vol. Ag. NO 3 added initial point after 0. 0 m. L of Ag. NO 3 added at the initial point of a titration of any type, only analyte is present, no titrant is present, therefore p. Ag can not be calculated. Dr. S. M. Condren

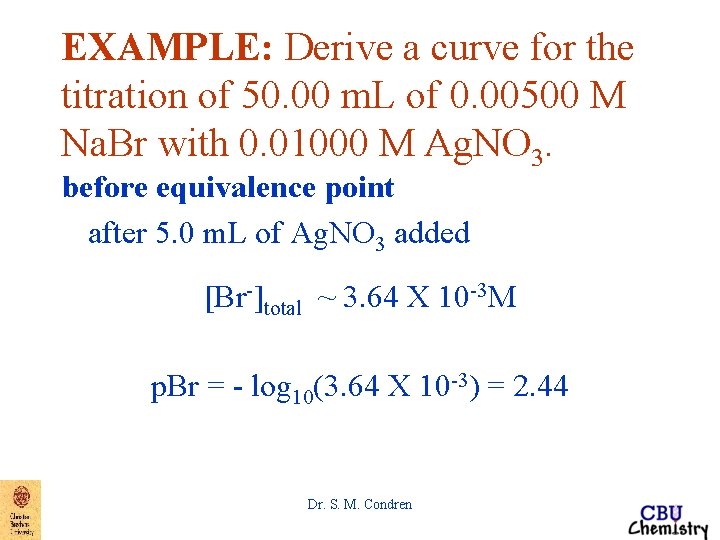
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point p. Ag can be accurately calculated only after some Ag. Br has started to form. This may take a few m. L of titrant Dr. S. M. Condren

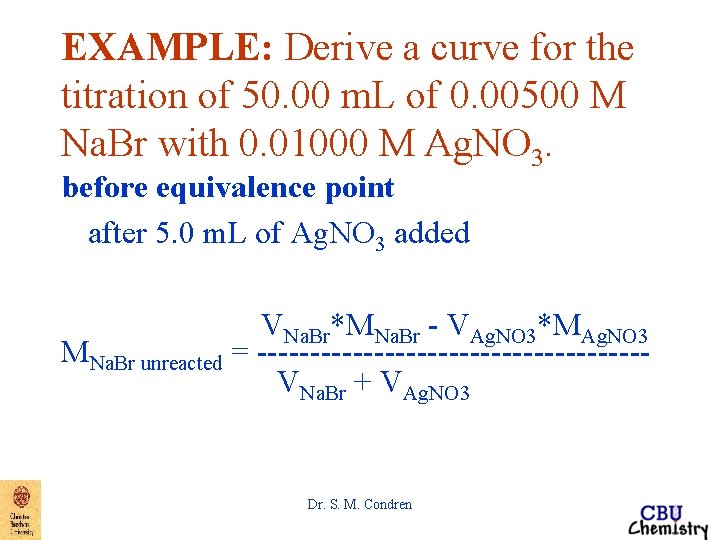
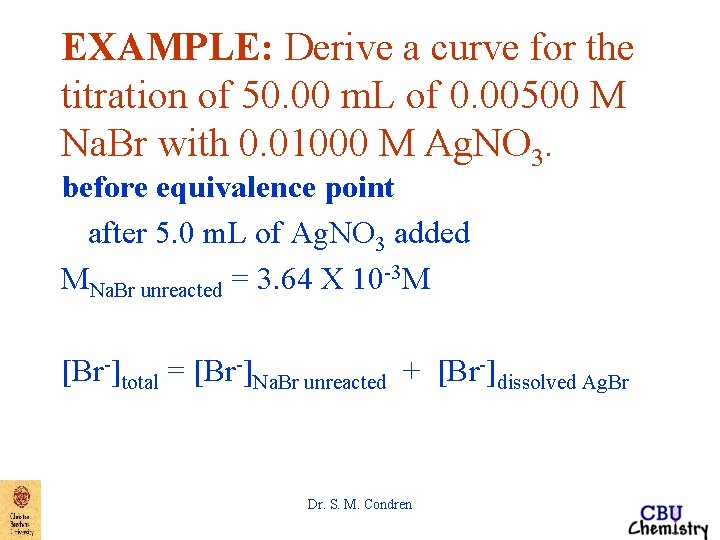
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added VNa. Br*MNa. Br - VAg. NO 3*MAg. NO 3 MNa. Br unreacted = ------------------VNa. Br + VAg. NO 3 Dr. S. M. Condren

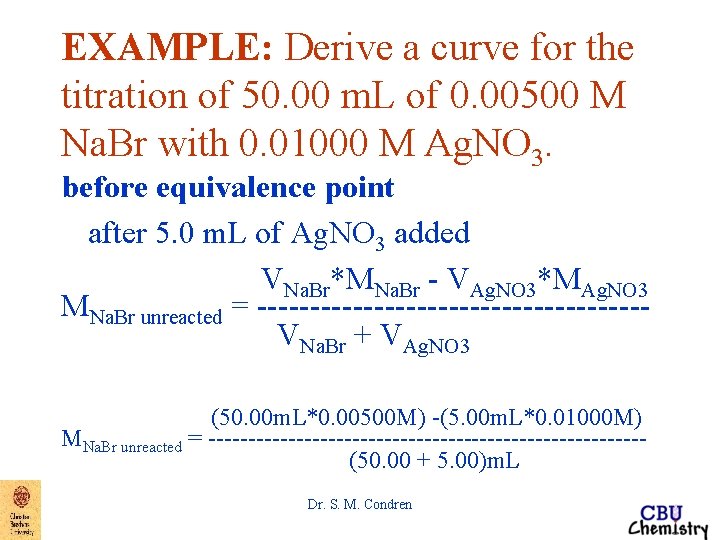
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added VNa. Br*MNa. Br - VAg. NO 3*MAg. NO 3 MNa. Br unreacted = ------------------VNa. Br + VAg. NO 3 (50. 00 m. L*0. 00500 M) -(5. 00 m. L*0. 01000 M) MNa. Br unreacted = ---------------------------(50. 00 + 5. 00)m. L Dr. S. M. Condren

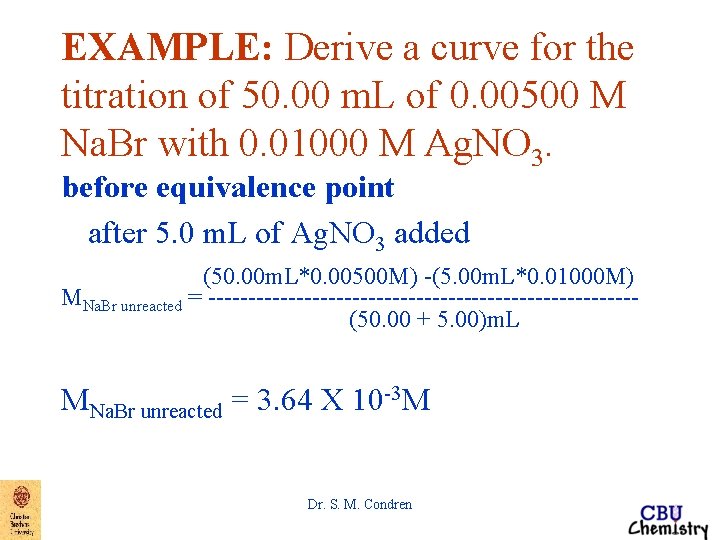
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added (50. 00 m. L*0. 00500 M) -(5. 00 m. L*0. 01000 M) MNa. Br unreacted = ---------------------------(50. 00 + 5. 00)m. L MNa. Br unreacted = 3. 64 X 10 -3 M Dr. S. M. Condren

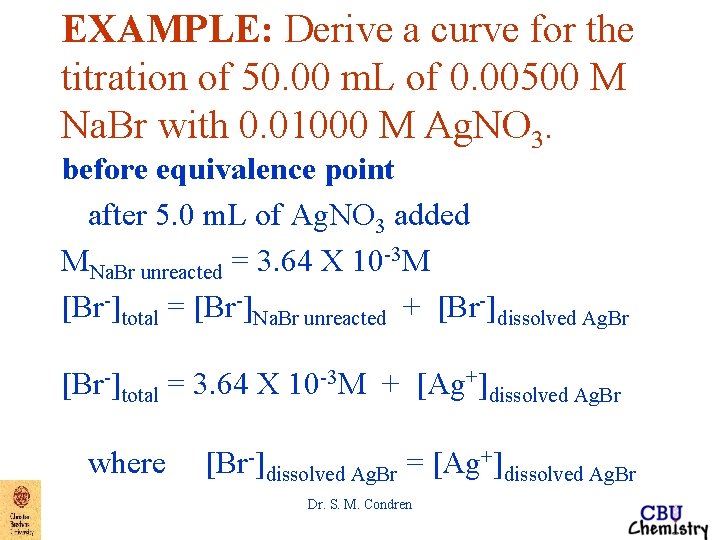
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added MNa. Br unreacted = 3. 64 X 10 -3 M [Br-]total = [Br-]Na. Br unreacted + [Br-]dissolved Ag. Br Dr. S. M. Condren

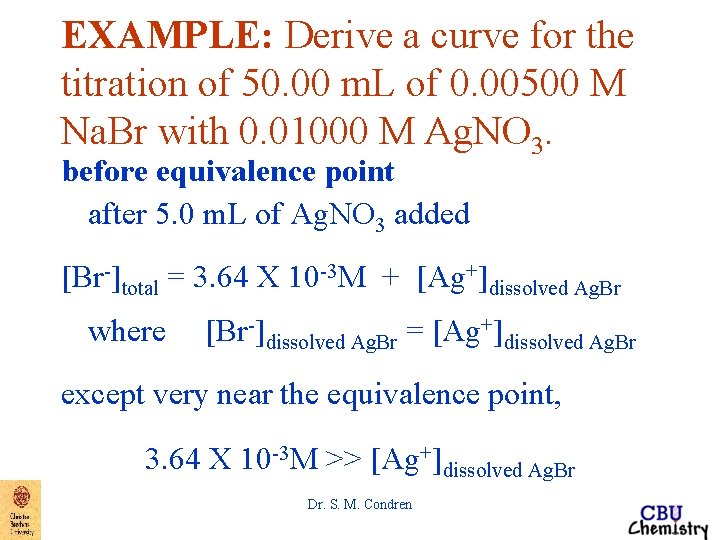
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added MNa. Br unreacted = 3. 64 X 10 -3 M [Br-]total = [Br-]Na. Br unreacted + [Br-]dissolved Ag. Br [Br-]total = 3. 64 X 10 -3 M + [Ag+]dissolved Ag. Br where [Br-]dissolved Ag. Br = [Ag+]dissolved Ag. Br Dr. S. M. Condren

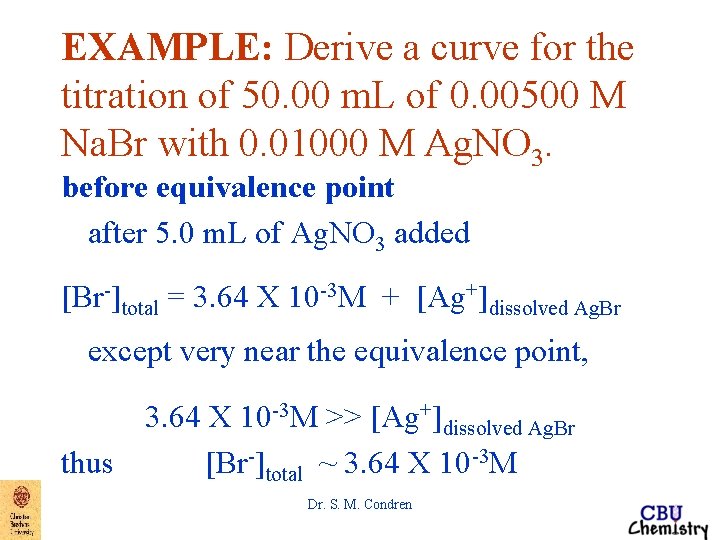
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added [Br-]total = 3. 64 X 10 -3 M + [Ag+]dissolved Ag. Br where [Br-]dissolved Ag. Br = [Ag+]dissolved Ag. Br except very near the equivalence point, 3. 64 X 10 -3 M >> [Ag+]dissolved Ag. Br Dr. S. M. Condren

EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added [Br-]total = 3. 64 X 10 -3 M + [Ag+]dissolved Ag. Br except very near the equivalence point, 3. 64 X 10 -3 M >> [Ag+]dissolved Ag. Br thus [Br-]total ~ 3. 64 X 10 -3 M Dr. S. M. Condren

EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added [Br-]total ~ 3. 64 X 10 -3 M p. Br = - log 10(3. 64 X 10 -3) = 2. 44 Dr. S. M. Condren

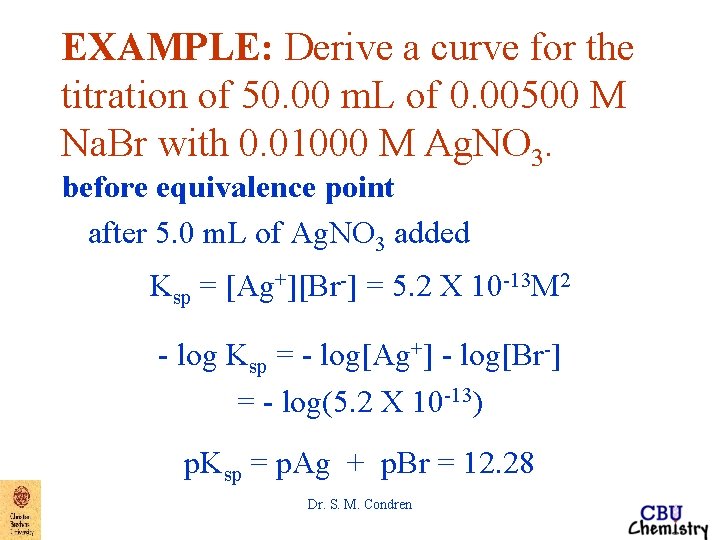
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added Ksp = [Ag+][Br-] = 5. 2 X 10 -13 M 2 - log Ksp = - log[Ag+] - log[Br-] = - log(5. 2 X 10 -13) p. Ksp = p. Ag + p. Br = 12. 28 Dr. S. M. Condren

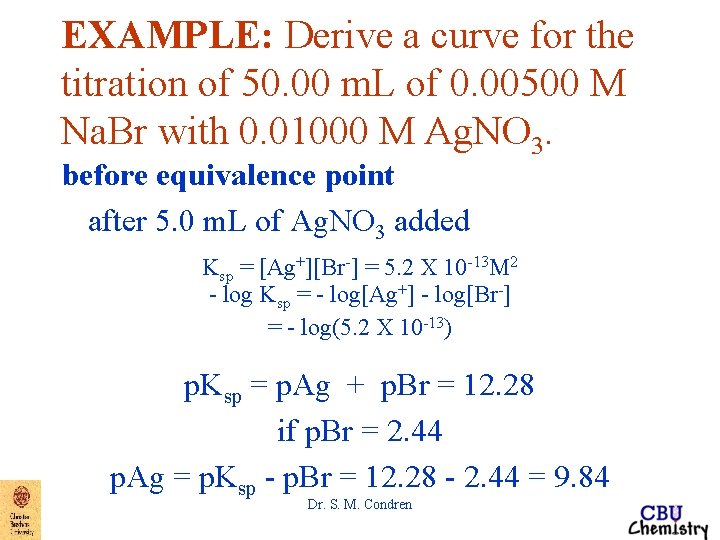
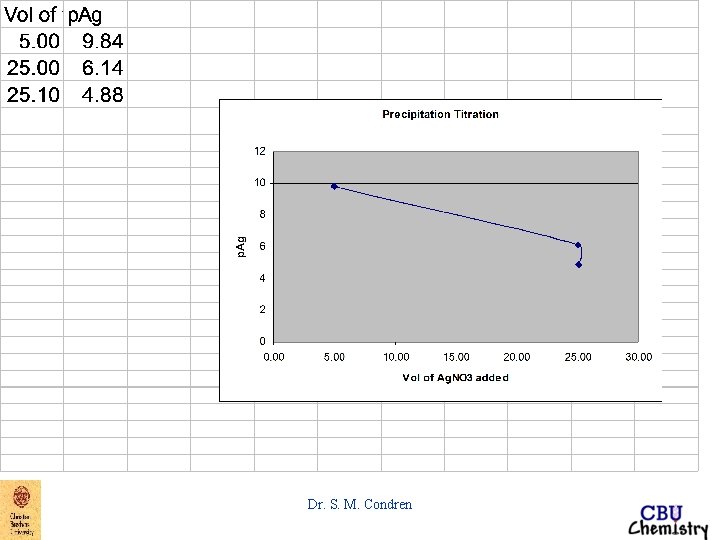
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. before equivalence point after 5. 0 m. L of Ag. NO 3 added Ksp = [Ag+][Br-] = 5. 2 X 10 -13 M 2 - log Ksp = - log[Ag+] - log[Br-] = - log(5. 2 X 10 -13) p. Ksp = p. Ag + p. Br = 12. 28 if p. Br = 2. 44 p. Ag = p. Ksp - p. Br = 12. 28 - 2. 44 = 9. 84 Dr. S. M. Condren

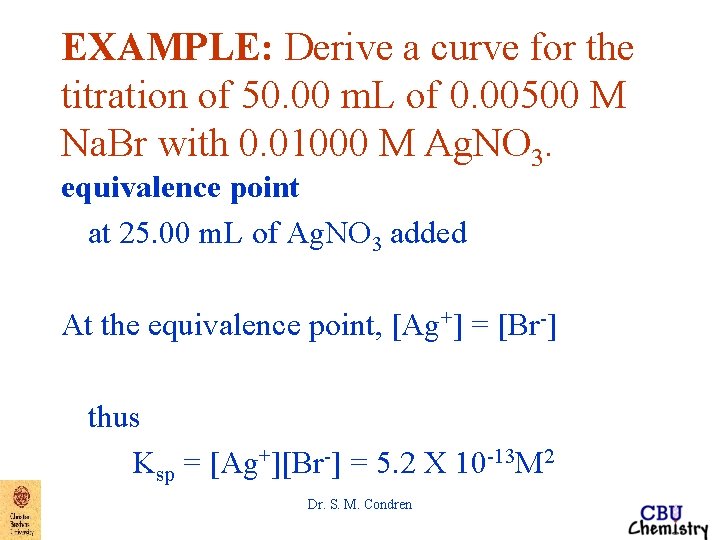
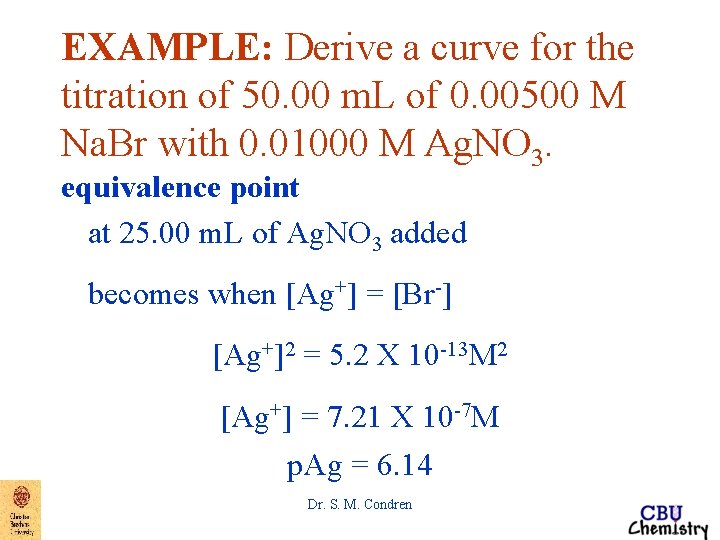
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. equivalence point at 25. 00 m. L of Ag. NO 3 added At the equivalence point, [Ag+] = [Br-] thus Ksp = [Ag+][Br-] = 5. 2 X 10 -13 M 2 Dr. S. M. Condren

EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. equivalence point at 25. 00 m. L of Ag. NO 3 added becomes when [Ag+] = [Br-] [Ag+]2 = 5. 2 X 10 -13 M 2 [Ag+] = 7. 21 X 10 -7 M p. Ag = 6. 14 Dr. S. M. Condren

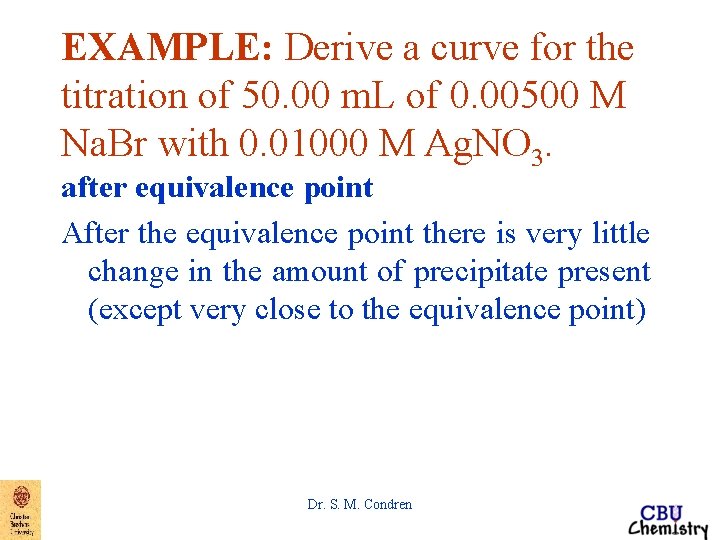
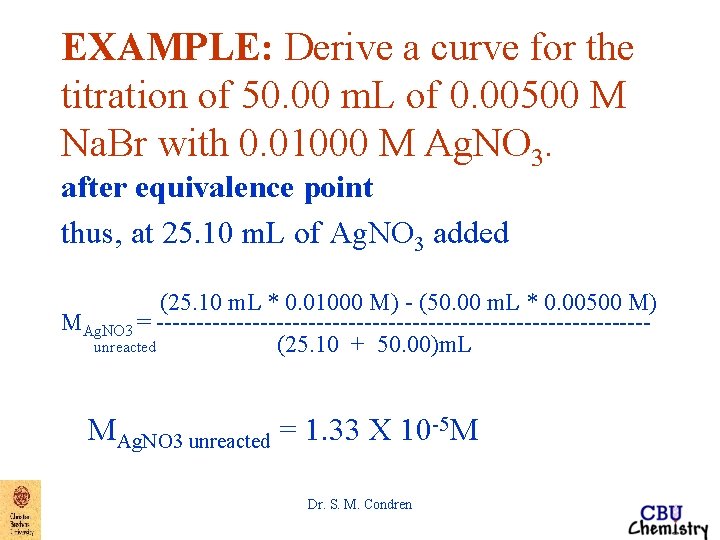
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. after equivalence point After the equivalence point there is very little change in the amount of precipitate present (except very close to the equivalence point) Dr. S. M. Condren

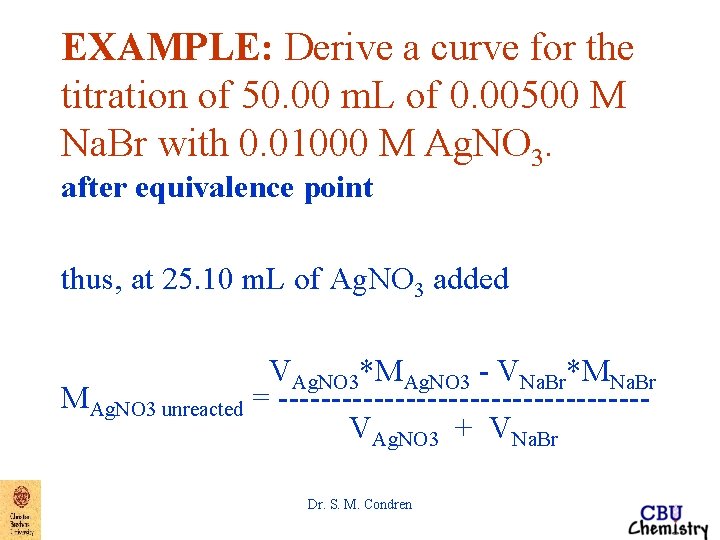
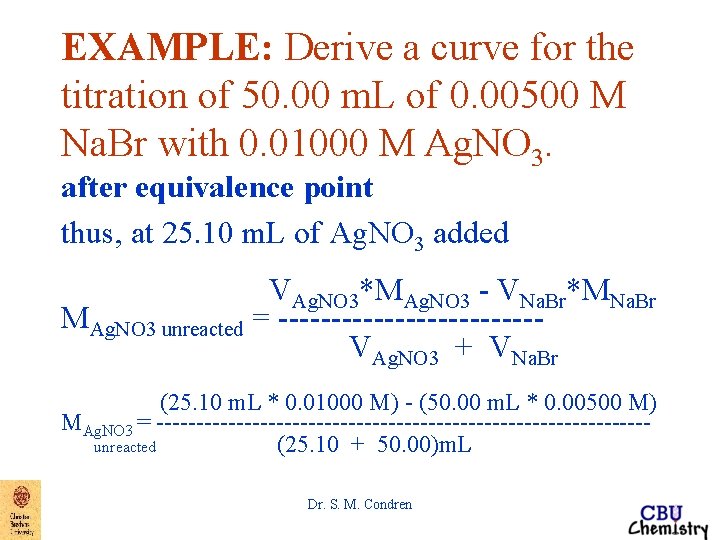
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. after equivalence point thus, at 25. 10 m. L of Ag. NO 3 added VAg. NO 3*MAg. NO 3 - VNa. Br*MNa. Br MAg. NO 3 unreacted = -----------------VAg. NO 3 + VNa. Br Dr. S. M. Condren

EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. after equivalence point thus, at 25. 10 m. L of Ag. NO 3 added VAg. NO 3*MAg. NO 3 - VNa. Br*MNa. Br MAg. NO 3 unreacted = ------------VAg. NO 3 + VNa. Br (25. 10 m. L * 0. 01000 M) - (50. 00 m. L * 0. 00500 M) MAg. NO 3 = -------------------------------unreacted (25. 10 + 50. 00)m. L Dr. S. M. Condren

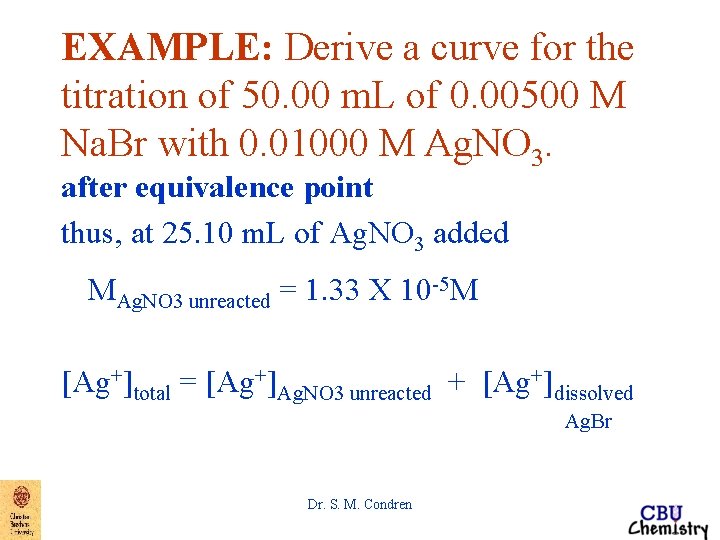
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. after equivalence point thus, at 25. 10 m. L of Ag. NO 3 added (25. 10 m. L * 0. 01000 M) - (50. 00 m. L * 0. 00500 M) MAg. NO 3 = -------------------------------unreacted (25. 10 + 50. 00)m. L MAg. NO 3 unreacted = 1. 33 X 10 -5 M Dr. S. M. Condren

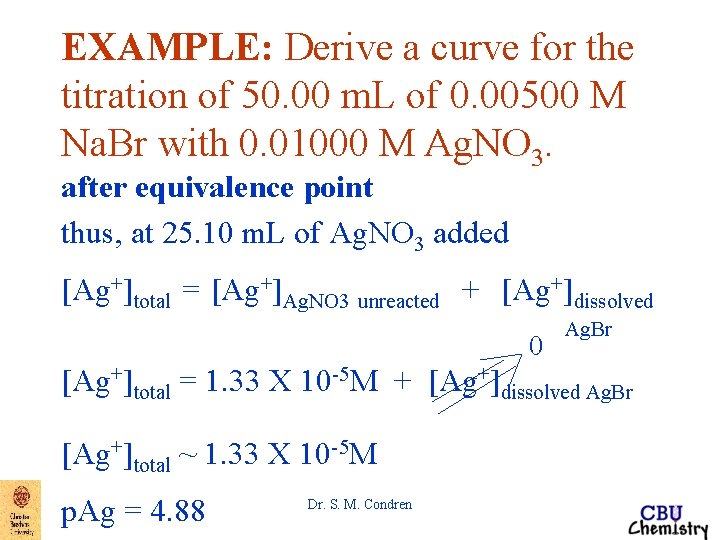
EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. after equivalence point thus, at 25. 10 m. L of Ag. NO 3 added MAg. NO 3 unreacted = 1. 33 X 10 -5 M [Ag+]total = [Ag+]Ag. NO 3 unreacted + [Ag+]dissolved Ag. Br Dr. S. M. Condren

EXAMPLE: Derive a curve for the titration of 50. 00 m. L of 0. 00500 M Na. Br with 0. 01000 M Ag. NO 3. after equivalence point thus, at 25. 10 m. L of Ag. NO 3 added [Ag+]total = [Ag+]Ag. NO 3 unreacted + [Ag+]dissolved 0 Ag. Br [Ag+]total = 1. 33 X 10 -5 M + [Ag+]dissolved Ag. Br [Ag+]total ~ 1. 33 X 10 -5 M p. Ag = 4. 88 Dr. S. M. Condren

Dr. S. M. Condren

Dr. S. M. Condren

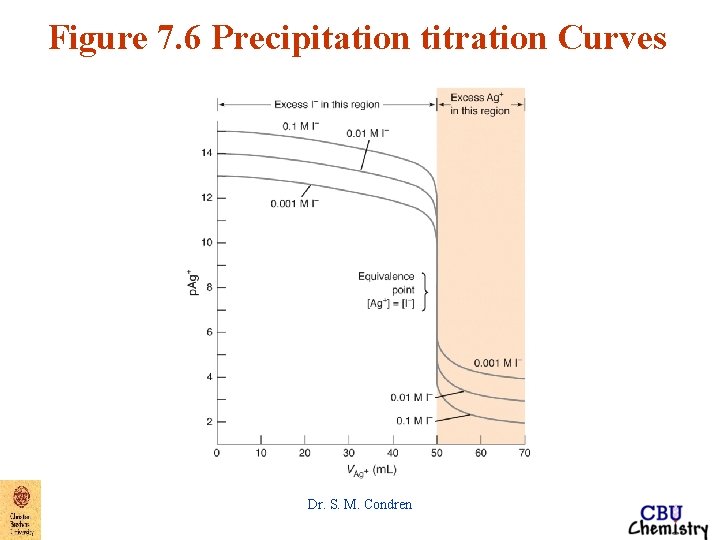
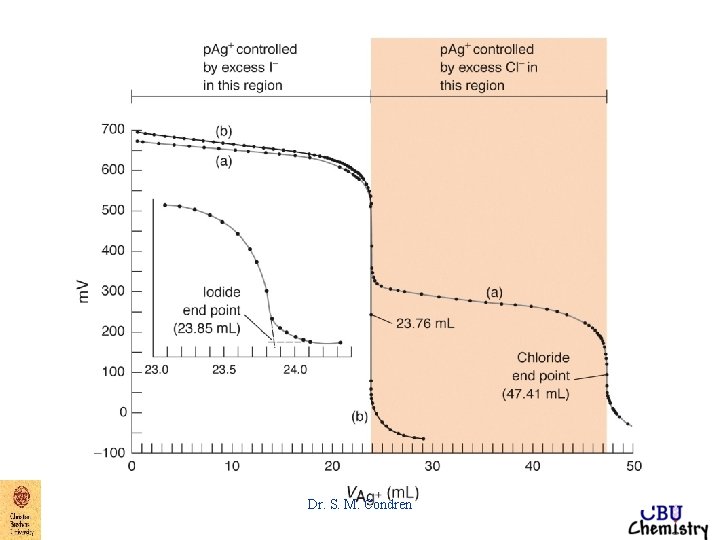
Figure 7. 6 Precipitation titration Curves Dr. S. M. Condren

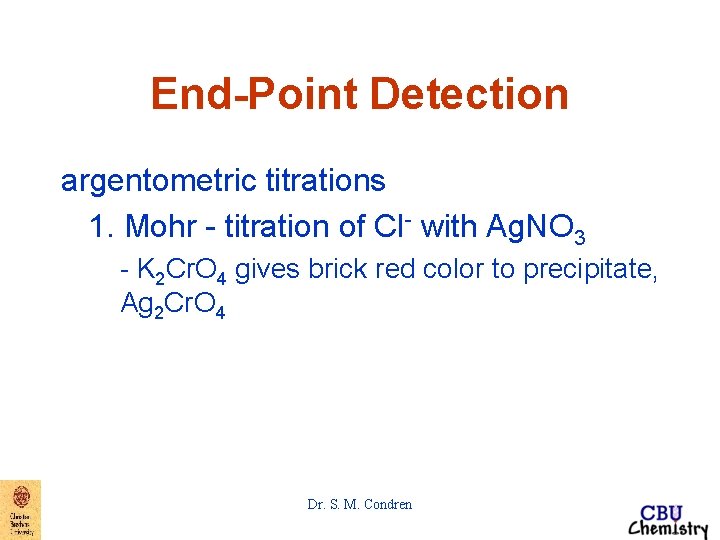
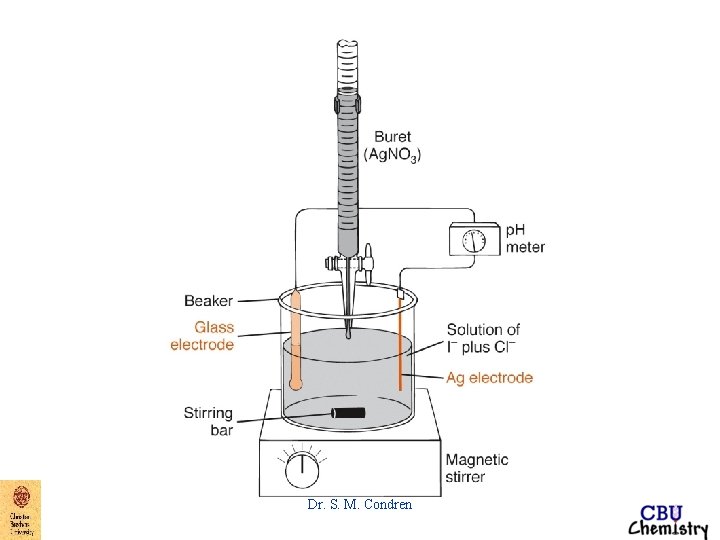
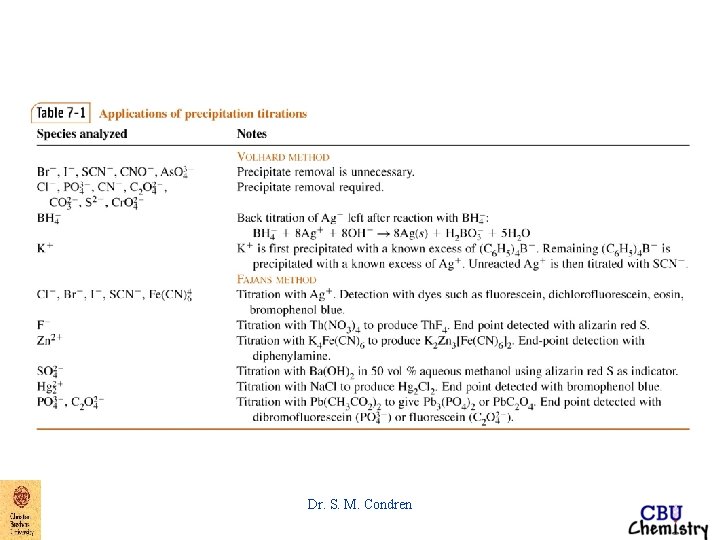
End-Point Detection argentometric titrations 1. Mohr - titration of Cl- with Ag. NO 3 - K 2 Cr. O 4 gives brick red color to precipitate, Ag 2 Cr. O 4 Dr. S. M. Condren

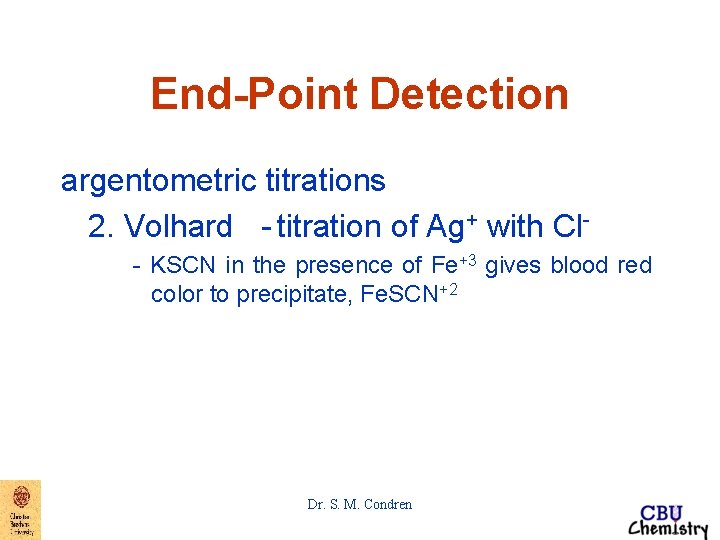
End-Point Detection argentometric titrations 2. Volhard - titration of Ag+ with Cl- KSCN in the presence of Fe+3 gives blood red color to precipitate, Fe. SCN+2 Dr. S. M. Condren

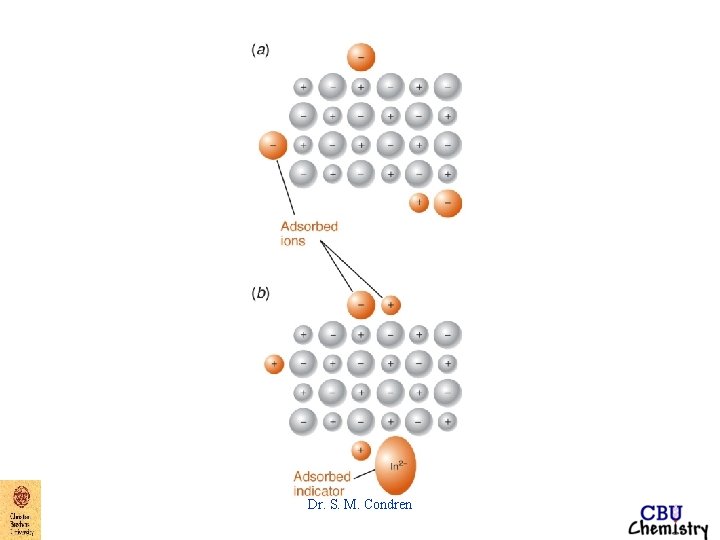
End-Point Detection argentometric titrations 3. Fajans - titration of Cl- with Ag. NO 3 - absorption indicator, dye absorbs on to the surface of the particles of Ag. Cl, attracted by excess Cl- absorbed on the surface of the precipitate Dr. S. M. Condren

Dr. S. M. Condren

Dr. S. M. Condren

Dr. S. M. Condren

Dr. S. M. Condren

Dr. S. M. Condren

Dr. S. M. Condren