Useful revision guide Instant Clinical Pharmacology E J









- Slides: 23

Useful revision guide Instant Clinical Pharmacology E. J. Begg Useful information on individual drugs (although a bit old now) Basic Clinical Pharmacokinetics (2 nd Edition) M. E. Winter

Drug dosing Important factors • concentration of drug in plasma • rate of drug elimination • rate of drug absorption

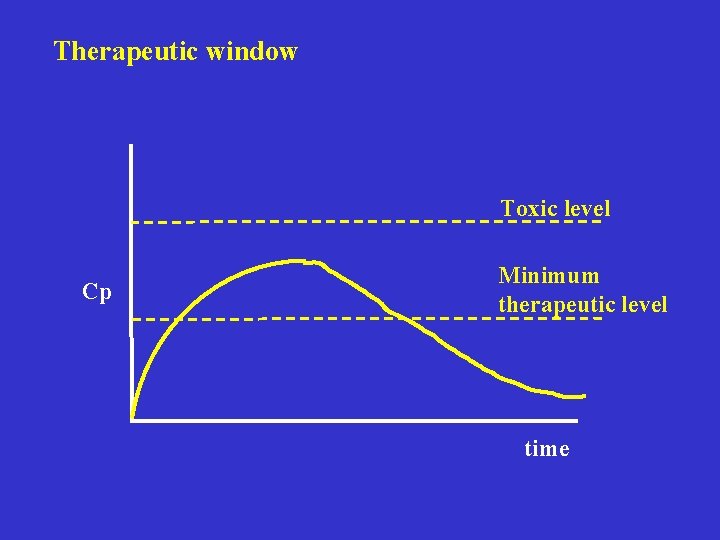
Therapeutic window Toxic level Cp Minimum therapeutic level time

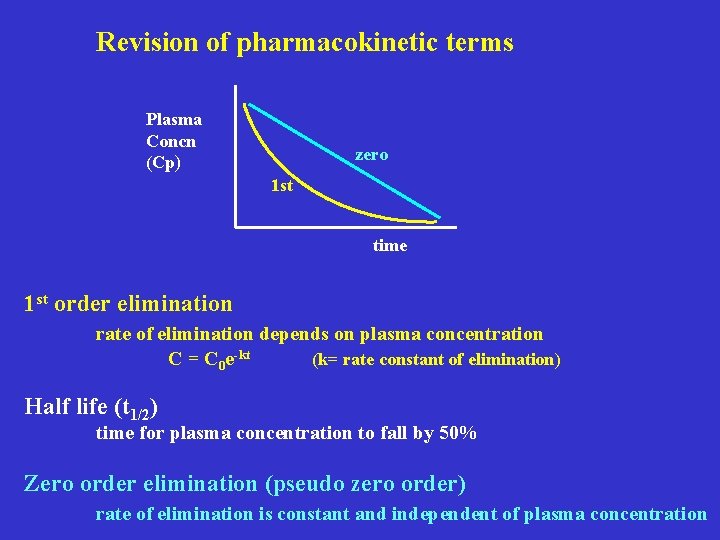
Revision of pharmacokinetic terms Plasma Concn (Cp) zero 1 st time 1 st order elimination rate of elimination depends on plasma concentration C = C 0 e-kt (k= rate constant of elimination) Half life (t 1/2) time for plasma concentration to fall by 50% Zero order elimination (pseudo zero order) rate of elimination is constant and independent of plasma concentration

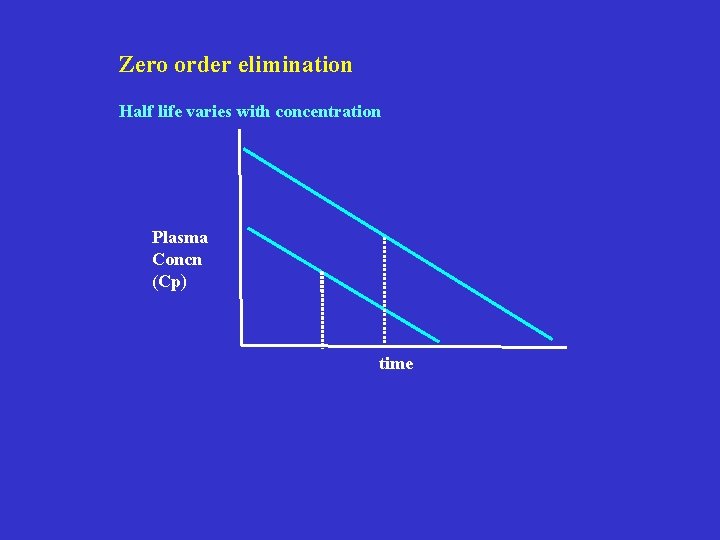
Zero order elimination Half life varies with concentration Plasma Concn (Cp) time

Volume of distribution (Vd) Vd = dose C 0 Volume of water in which a drug would have to be distributed to give its plasma concentration at time zero. Can be larger than total body volume frusemide 7 litres aspirin 14 litres propranolol 273 litres digitoxin 38 liters 4 ml min-1 digoxin 640 litres 130 ml min -1 Plasma clearance (Cl. P) volume of blood cleared of its drug content in unit time CP= Cl. M + Cl. R + Cl. B + …….

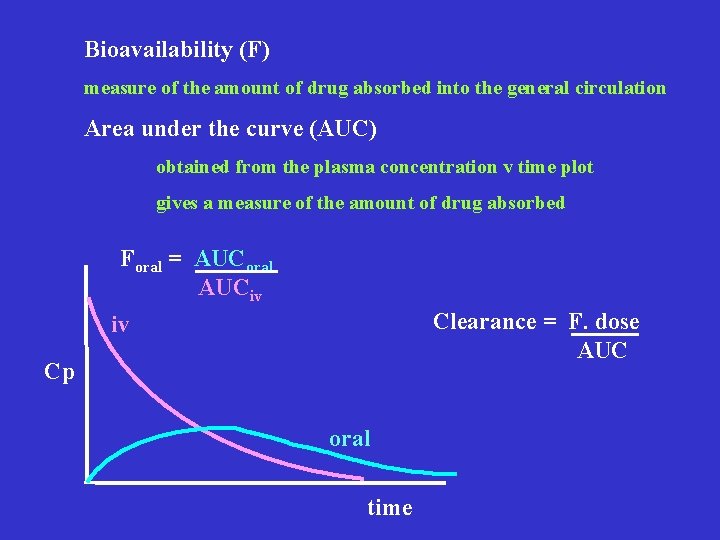
Bioavailability (F) measure of the amount of drug absorbed into the general circulation Area under the curve (AUC) obtained from the plasma concentration v time plot gives a measure of the amount of drug absorbed Foral = AUCoral AUCiv Clearance = F. dose AUC iv Cp oral time

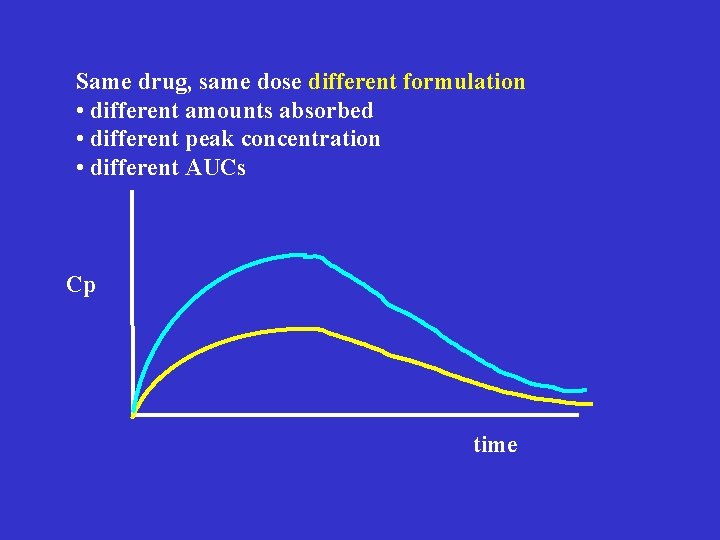
Same drug, same dose different formulation • different amounts absorbed • different peak concentration • different AUCs Cp time

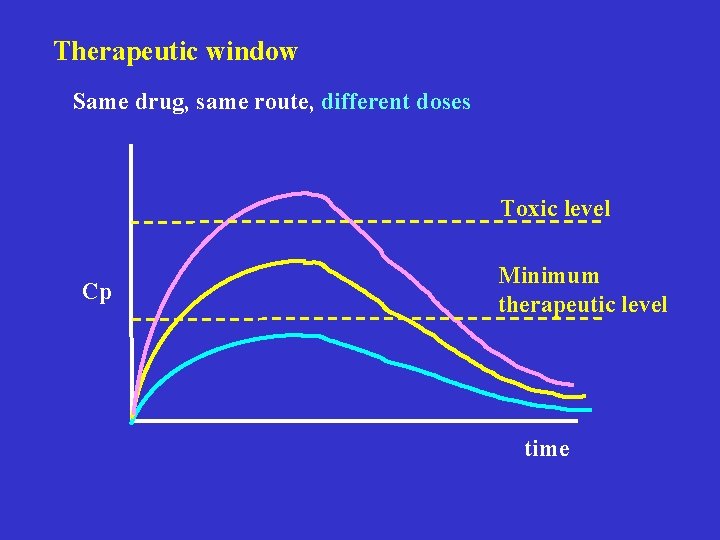
Therapeutic window Same drug, same route, different doses Toxic level Cp Minimum therapeutic level time

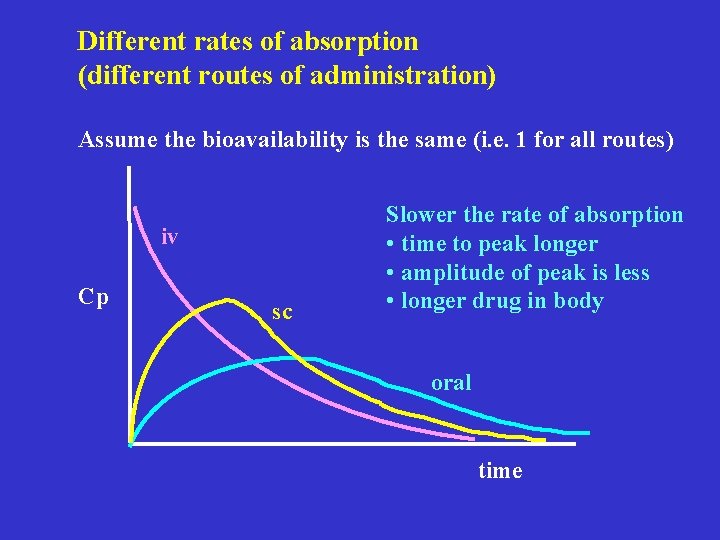
Different rates of absorption (different routes of administration) Assume the bioavailability is the same (i. e. 1 for all routes) iv Cp sc Slower the rate of absorption • time to peak longer • amplitude of peak is less • longer drug in body oral time

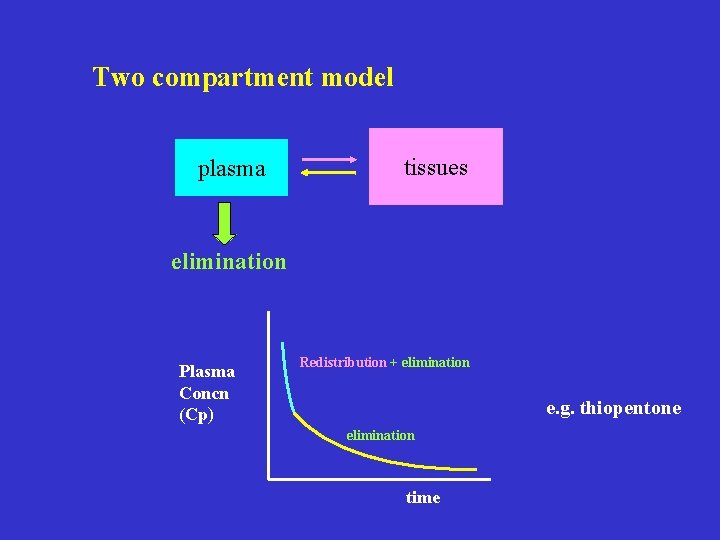
Two compartment model plasma tissues elimination Plasma Concn (Cp) Redistribution + elimination e. g. thiopentone elimination time

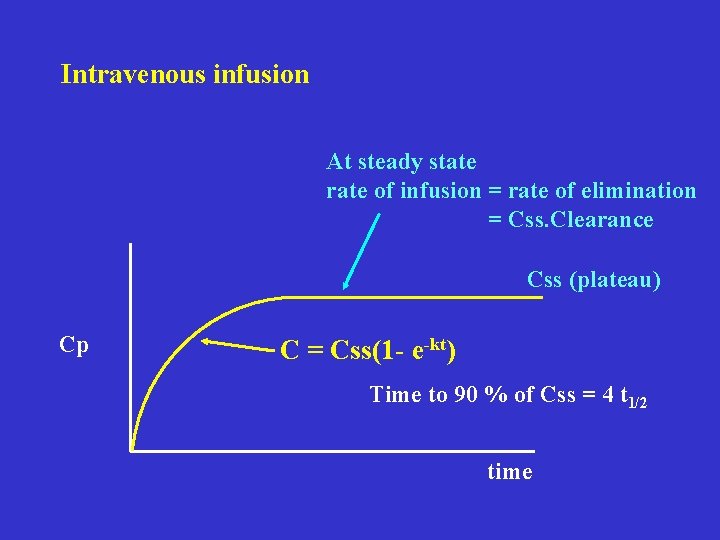
Intravenous infusion At steady state rate of infusion = rate of elimination = Css. Clearance Css (plateau) Cp C = Css(1 - e-kt) Time to 90 % of Css = 4 t 1/2 time

Half life hours steady state Lignocaine 2 8 hours Valproate 6 24 hours Digoxin 32 6 days Digitoxin 161 28 days

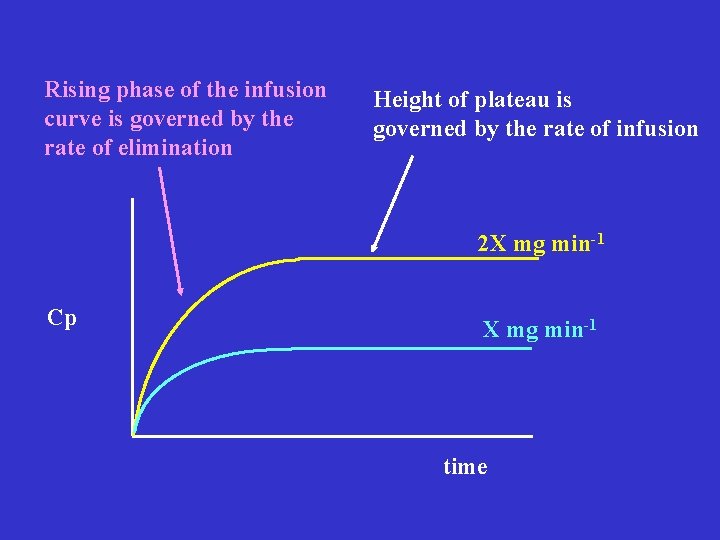
Rising phase of the infusion curve is governed by the rate of elimination Height of plateau is governed by the rate of infusion 2 X mg min-1 Cp X mg min-1 time

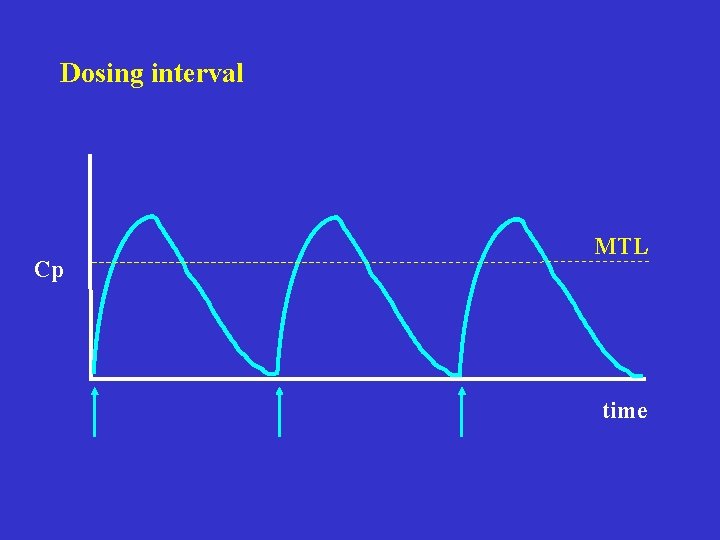
Dosing interval Cp MTL time

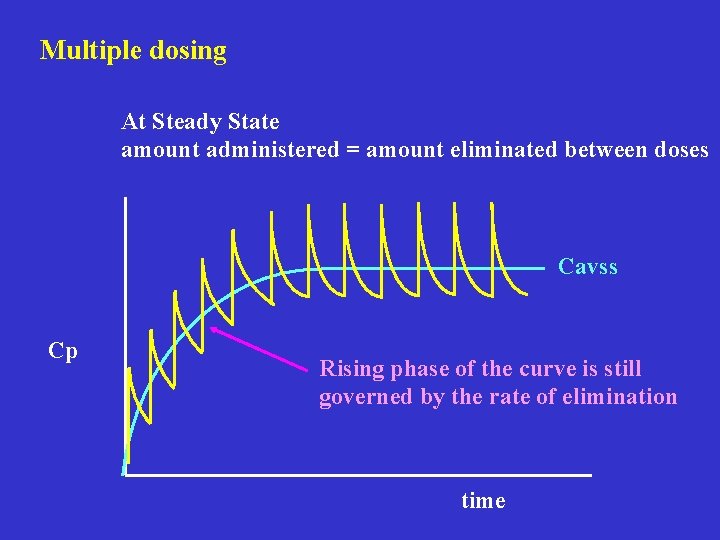
Multiple dosing At Steady State amount administered = amount eliminated between doses Cavss Cp Rising phase of the curve is still governed by the rate of elimination time

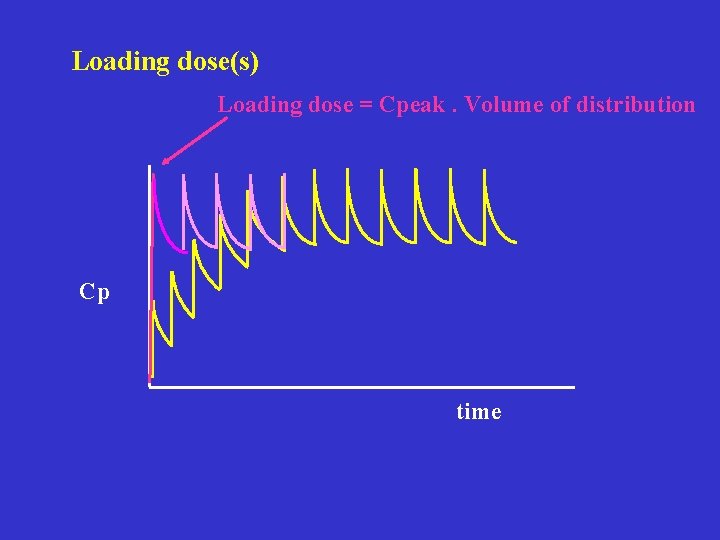
Loading dose(s) Loading dose = Cpeak. Volume of distribution Cp time

Tetracycline t 1/2 = 8 hours 500 mg loading dose followed by 250 mg every 8 hours

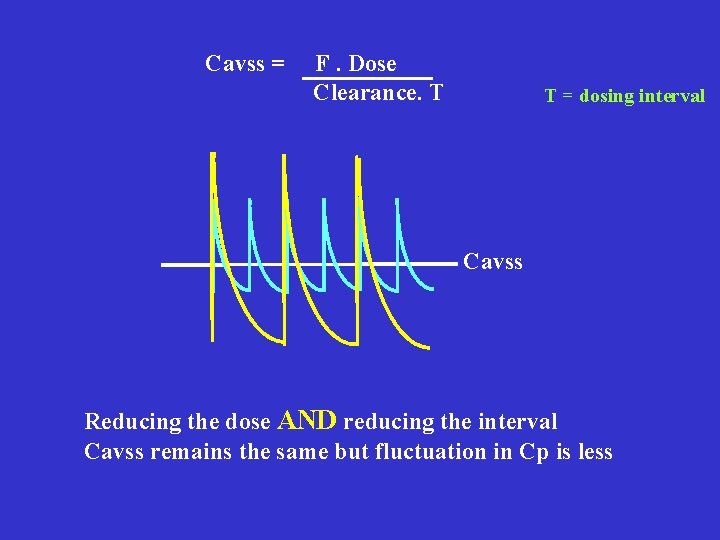
Cavss = F. Dose Clearance. T T = dosing interval Cavss Reducing the dose AND reducing the interval Cavss remains the same but fluctuation in Cp is less

Drug plasma concentration monitoring is helpful for drugs • that have a low therapeutic index • that are not metabolized to active metabolites • whose concentration is not predictable from the dose • whose concentration relates well to eitherapeutic effect or the toxic effect, and preferably both • that are often taken in overdose

For which specific drugs is drug concentration monitoring helpful? The important drugs are: • aminoglycoside antibiotics (plasma or serum) • ciclosporin (whole blood) • digoxin and digitoxin (plasma or serum) • lithium (serum) • phenytoin (plasma or serum) • theophylline (plasma or serum) • paracetamol and salicylate (overdose) (plasma or serum). Other drugs are sometimes measured: • anticonvulsants other than phenytoin (eg carbamazepine, valproate) • tricyclic antidepressants (especially nortriptyline) • anti-arrhythmic drugs (eg amiodarone).

The uses of monitoring are • to assess adherence to therapy • to individualize therapy • to diagnose toxicity • to guide withdrawal of therapy • to determine whether a patient is already taking a drug before starting therapy (eg theophylline in an unconscious patient with asthma) • in research (eg to monitor for drug interactions in post-marketing surveillance using population pharmacokinetics).

Altered pharmacokinetic profile • liver metabolism Disease Pharmacogenetics (cytochrome P 450 polymorphisms) • renal impairment Disease Elderly
 Clinical pharmacology
Clinical pharmacology Clinical pharmacology residency
Clinical pharmacology residency Basic & clinical pharmacology
Basic & clinical pharmacology Clinical pharmacology seminar
Clinical pharmacology seminar Dopamine synthesis
Dopamine synthesis Clinical pharmacology seminar
Clinical pharmacology seminar Clinical pharmacology powered by clinicalkey
Clinical pharmacology powered by clinicalkey Chronic gout
Chronic gout Passive voice revision
Passive voice revision Stout motherly mare meaning
Stout motherly mare meaning Crime and punishment revision guide
Crime and punishment revision guide Aqa maths gcse revision guide
Aqa maths gcse revision guide History gcse spec
History gcse spec Engineering revision guide
Engineering revision guide How to do clinical audit a brief guide
How to do clinical audit a brief guide Npte pharmacology
Npte pharmacology Pharmacology introduction
Pharmacology introduction Chapter 30 principles of pharmacology
Chapter 30 principles of pharmacology Fish pharmacology
Fish pharmacology Maintenance dose formula
Maintenance dose formula Samyukti meaning
Samyukti meaning Toxicology and applied pharmacology
Toxicology and applied pharmacology Pharmacology for nurses: a pathophysiological approach
Pharmacology for nurses: a pathophysiological approach Diazepa
Diazepa