Update from education committee Train the trainercontent reviewed










- Slides: 17

Update from education committee • Train the trainer—content reviewed from TIMELESS Clinical Trial “First Tuesdays” Lecture Series

Introduction and Goal of “First Tuesdays” • Sabreena Slavin MD – Vascular Neurologist and Neurohospitalist at KU School of Medicine • Didactic lecture series as part of the Kansas Initiative for Stroke Survival • Updates in Practice and FAQ’s on Acute Stroke Care • 20 minute didactic, 10 minutes for questions/discussion.


Review of Acute Stroke Medical Intervention • IV alteplase for all patients who have disabling symptoms of acute stroke • Time limit for IV alteplase: 4. 5 hours from last known normal per current stroke guidelines • Absolute contraindications: – Acute ICH – Currently on anticoagulation (can be on Warfarin if INR is < 1. 7) – Thrombocytopenia or significant coagulopathies

What about IV thrombolysis in those outside the window? • A PHASE III, PROSPECTIVE, DOUBLE-BLIND, RANDOMIZED, PLACEBO-CONTROLLED TRIAL OF THROMBOLYSIS IN IMAGING-ELIGIBLE, LATE -WINDOW PATIENTS TO ASSESS THE EFFICACY AND SAFETY OF TENECTEPLASE (TIMELESS)

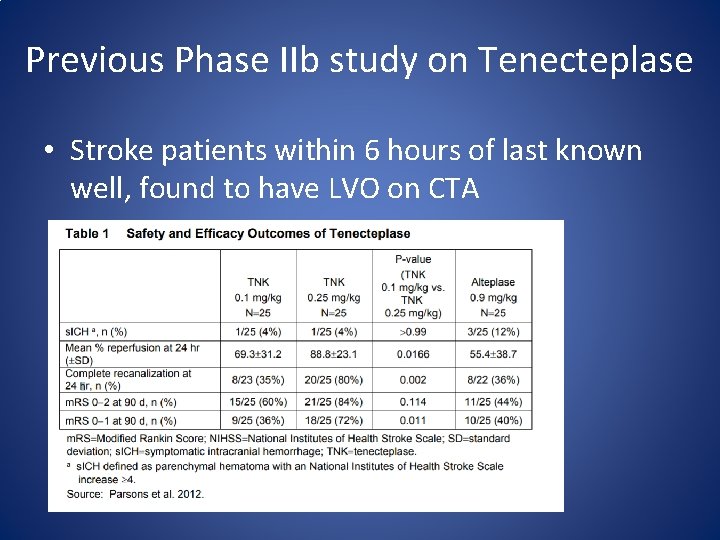
Previous Phase IIb study on Tenecteplase • Stroke patients within 6 hours of last known well, found to have LVO on CTA

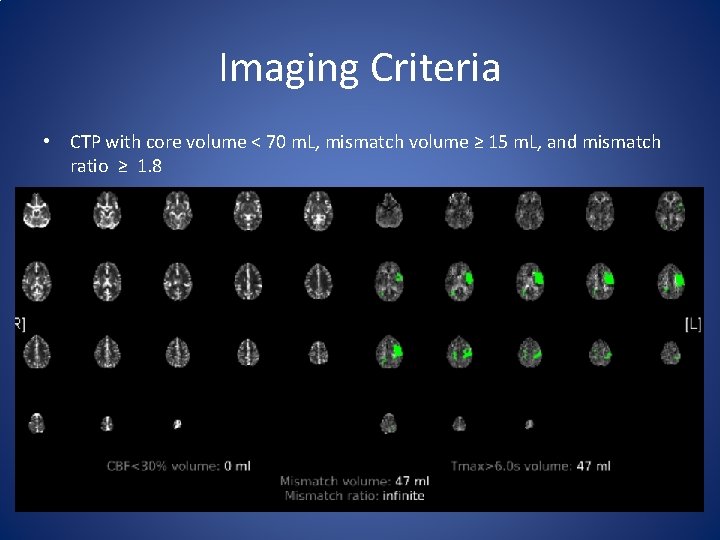
TIMELESS • Compare efficacy of Tenecteplase 0. 25 mg/kg (maximum 25 mg) vs placebo in acute ischemic stroke patients in 4. 5 - 24 hours from last well • Needs to have an ICA, M 1, or M 2 occlusion and NIHSS ≥ 5 • Needs to have CTP with core volume < 70 m. L, mismatch volume ≥ 15 m. L, and mismatch ratio ≥ 1. 8 • Primary outcome will be 90 day m. RS ordinal analysis • Plan to enroll 456 patients total

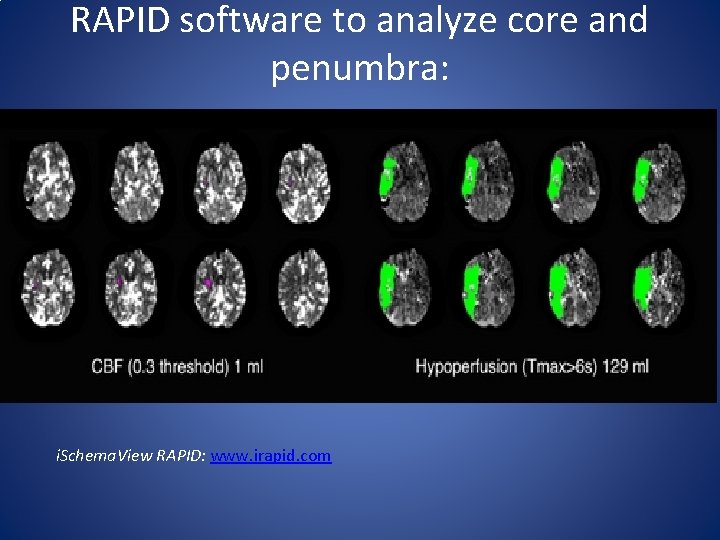
Perfusion Imaging • CT perfusion and MR perfusion imaging is used to find a mismatch between ischemic core (area already damaged) and ischemic penumbra (area at risk of damage). • Measures of core: cerebral blood volume, cerebral blood flow • Measures of penumbra: mean transit time (ratio cerebral blood flow/cerebral blood volume), time to peak, time to drain, and Tmax (measures of contrast arrival time to tissue).

RAPID software to analyze core and penumbra: i. Schema. View RAPID: www. irapid. com

Imaging Criteria • CTP with core volume < 70 m. L, mismatch volume ≥ 15 m. L, and mismatch ratio ≥ 1. 8

Other inclusion criteria • Age ≥ 18 • prestroke m. RS 0 -2

Exclusion criteria (similar to criteria for alteplase) • • • • Current participation in another investigational drug or device study Active internal bleeding Known hypersensitivity or allergy to any ingredients of tenecteplase Known bleeding diathesis Known hereditary or acquired hemorrhagic diathesis, coagulation factor deficiency; recent oral anticoagulant therapy with INR ≥ 1. 7 Use of one of the new oral anticoagulants within the last 48 hours (dabigatran, rivaroxaban, apixaban) Pregnant Intracranial neoplasm, arteriovenous malformation, or aneurysm Seizures at stroke onset if it precludes obtaining an accurate baseline NIHSS Severe, uncontrolled hypertension (systolic blood pressure ≥ 180 mm. Hg or diastolic blood pressure ≥ 110 mm. Hg) Baseline platelet count ≥ 100, 000/μL (results must be available prior to treatment) Baseline blood glucose ≥ 400 mg/d. L (22. 20 mmol/L) Baseline blood glucose ≤ 50 mg/d. L needs to be normalized prior to randomization

Exclusion criteria, continued • Clot retrieval attempted using a neuro-thrombectomy device prior to randomization • Intracranial or intraspinal surgery or trauma within 2 months • Treatment with a thrombolytic within the last 3 months prior to randomization • Other serious, advanced, or terminal illness (investigator judgment) or life expectancy is less than 6 months • Pre-existing medical, neurological, or psychiatric disease that would confound the neurological or functional evaluations • History of cerebrovascular accident in the last 90 days or any history of intracerebral hemorrhage • Presumed septic embolus; suspicion of bacterial endocarditis

Exclusion criteria, continued • Any other condition that, in the opinion of the investigator, precludes an endovascular procedure or poses a significant hazard to the patient if an endovascular procedure was to be performed • Extensive early ischemic change (hypodensity) on non-contrast CT estimated to be ≥ 1/3 MCA territory, or significant hypodensity outside the Tmax ≥ 6 s perfusion lesion that invalidates mismatch criteria (if patient is enrolled based on CT perfusion criteria) • Significant mass effect • Acute symptomatic arterial occlusions in more than one vascular territory confirmed on CTA/MRA (e. g. , bilateral MCA occlusions, or an MCA and a basilar artery occlusion) • Evidence of intracranial tumor (except small meningioma) acute intracranial hemorrhage, neoplasm, or arteriovenous malformation • Carotid stenting or requiring carotid reconstruction in the setting of severe dissection

Telephone consent • Will discuss with decision-maker on phone about the study and send the consent form to their email • Will be able to enroll patient into the study before family arrives at KUMC if they are a candidate per imaging criteria

Reminders • If patient is out of time window for IV alteplase, they may still be able to receive IV treatment for their stroke through TIMELESS study. • Study drug will only be given at study site (KUMC), but imaging can be done at local ED (need CTA and CTP with RAPID). • Patients can still get endovascular therapy with the study.

Questions? • • Call for help anytime! http: //www. kissnetwork. us/ KU BAT phone: 913 -588 -3727 sslavin 2@kumc. edu