Tropospheric Ozone Chemistry David Plummer presented at the
















- Slides: 36

Tropospheric Ozone Chemistry David Plummer presented at the GCC Summer School Montreal, August 7 -13, 2003 Outline: - Solar radiation and chemistry - Tropospheric ozone production - Methane oxidation cycle - Nitrogen species - A look at global tropospheric ozone - Oxidizing capacity of the troposphere

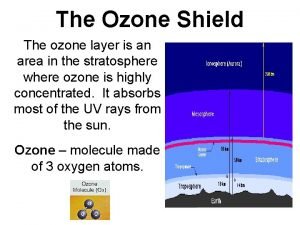
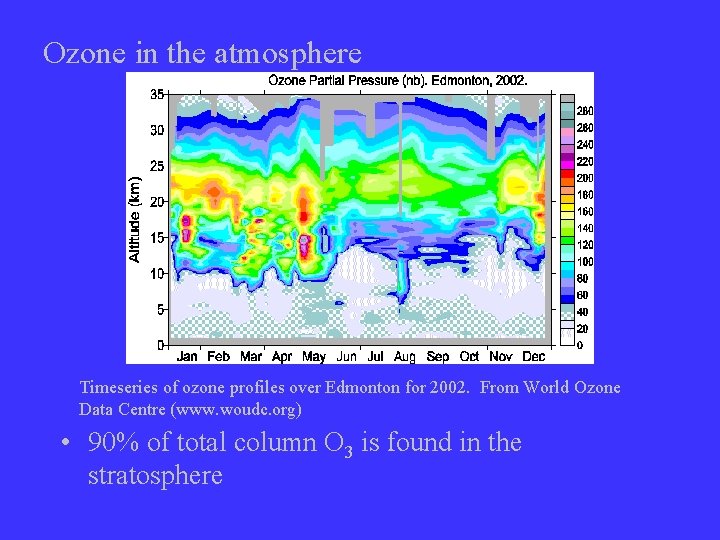
Ozone in the atmosphere Timeseries of ozone profiles over Edmonton for 2002. From World Ozone Data Centre (www. woudc. org) • 90% of total column O 3 is found in the stratosphere

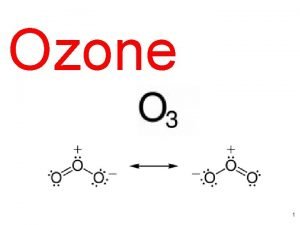
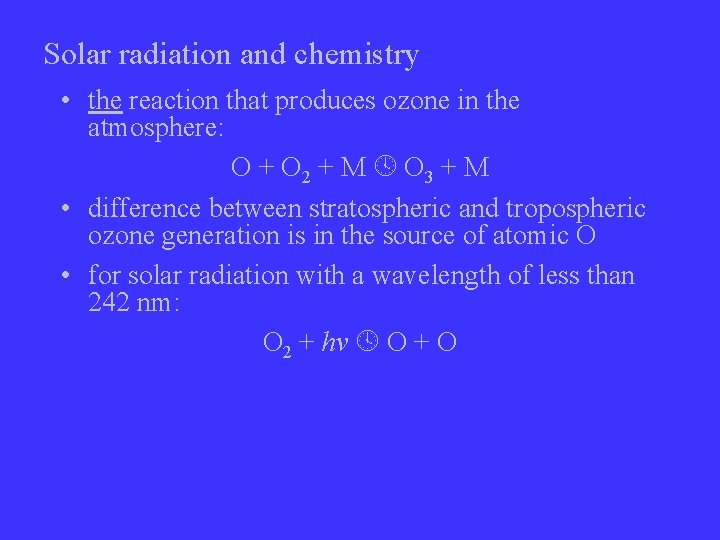
Solar radiation and chemistry • the reaction that produces ozone in the atmosphere: O + O 2 + M O 3 + M • difference between stratospheric and tropospheric ozone generation is in the source of atomic O • for solar radiation with a wavelength of less than 242 nm: O 2 + hv O + O

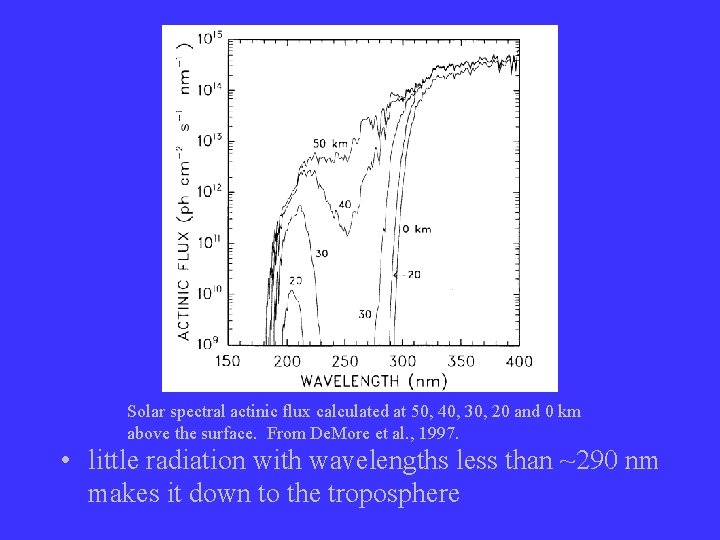
Solar spectral actinic flux calculated at 50, 40, 30, 20 and 0 km above the surface. From De. More et al. , 1997. • little radiation with wavelengths less than ~290 nm makes it down to the troposphere

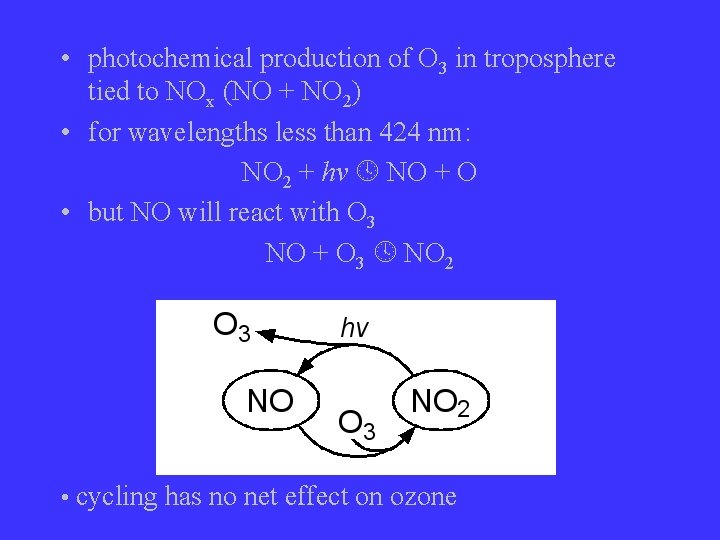
• photochemical production of O 3 in troposphere tied to NOx (NO + NO 2) • for wavelengths less than 424 nm: NO 2 + hv NO + O • but NO will react with O 3 NO + O 3 NO 2 • cycling has no net effect on ozone

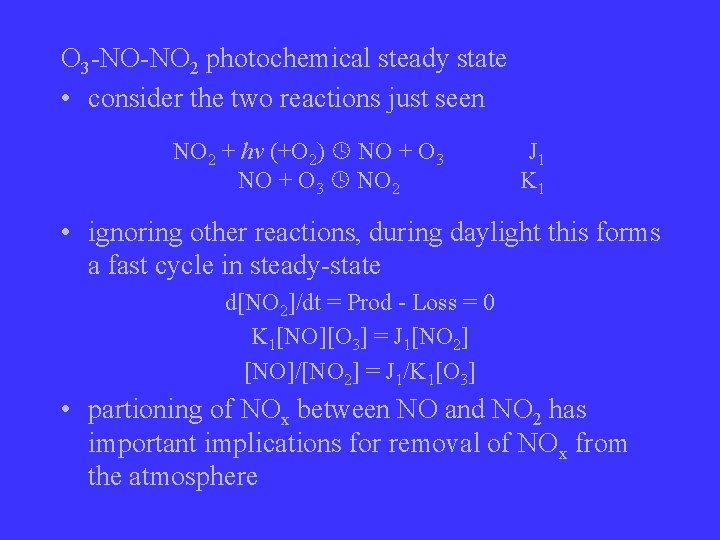
O 3 -NO-NO 2 photochemical steady state • consider the two reactions just seen NO 2 + hv (+O 2) NO + O 3 NO 2 J 1 K 1 • ignoring other reactions, during daylight this forms a fast cycle in steady-state d[NO 2]/dt = Prod - Loss = 0 K 1[NO][O 3] = J 1[NO 2] [NO]/[NO 2] = J 1/K 1[O 3] • partioning of NOx between NO and NO 2 has important implications for removal of NOx from the atmosphere

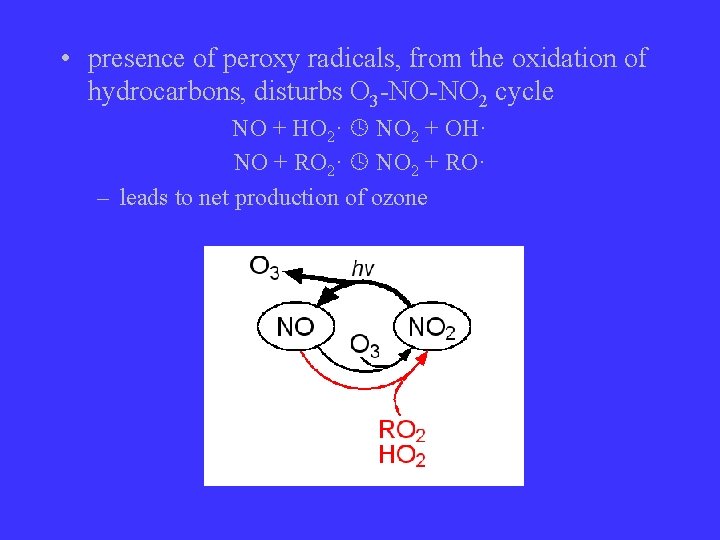
• presence of peroxy radicals, from the oxidation of hydrocarbons, disturbs O 3 -NO-NO 2 cycle NO + HO 2· NO 2 + OH· NO + RO 2· NO 2 + RO· – leads to net production of ozone

The Hydroxyl Radical • produced from ozone photolysis – for radiation with wavelengths less than 320 nm: O 3 + hv O(1 D) + O 2 followed by O(1 D) + M O(3 P) + M (+O 2 O 3) O(1 D) + H 2 O 2 OH· (~90%) (~10%) • OH initiates the atmospheric oxidation of a wide range of compounds in the atmosphere – referred to as ‘detergent of the atmosphere’ – typical concentrations near the surface ~106 - 107 cm-3 – very reactive, effectively recycled

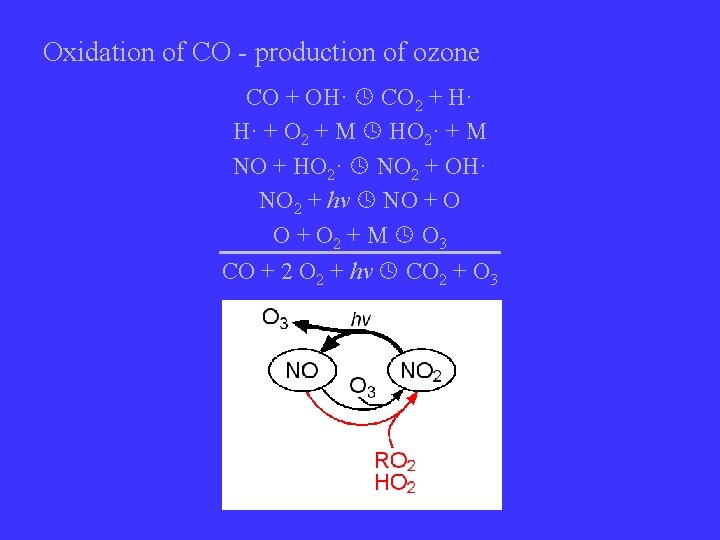
Oxidation of CO - production of ozone CO + OH· CO 2 + H· H· + O 2 + M HO 2· + M NO + HO 2· NO 2 + OH· NO 2 + hv NO + O 2 + M O 3 CO + 2 O 2 + hv CO 2 + O 3

What breaks the cycle? • cycle terminated by OH· + NO 2 HNO 3 HO 2· + HO 2· H 2 O 2 • both HNO 3 and H 2 O 2 will photolyze or react with OH to, in effect, reverse these pathways – but reactions are slow (lifetime of several days) – both are very soluble - though H 2 O 2 less-so • washout by precipitation • dry deposition – in PBL they are effectively a loss – situation is more complicated in the upper troposphere • no dry deposition, limited wet removal

Methane Oxidation Cycle • CH 4 is simplest alkane species – features of oxidation cycle common to other organic compounds • long photochemical lifetime – fairly evenly distributed throughout troposphere – concentrations ~1. 8 ppmv • reactions form ‘bedrock’ of the chemistry in the background troposphere

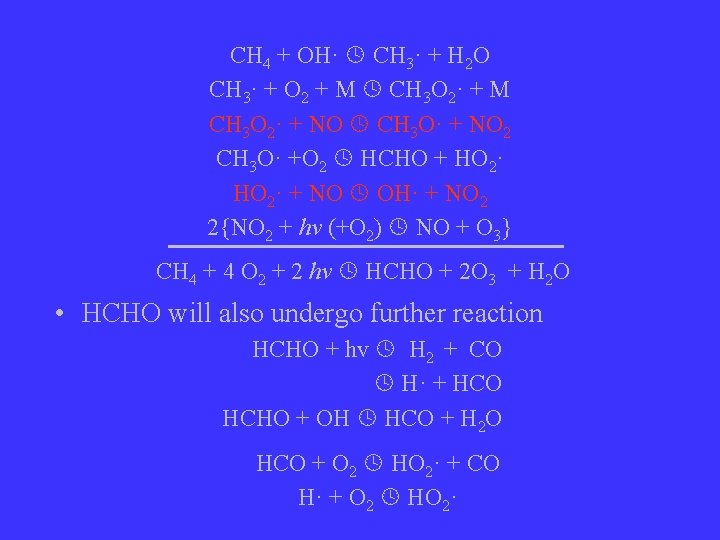
CH 4 + OH· CH 3· + H 2 O CH 3· + O 2 + M CH 3 O 2· + M CH 3 O 2· + NO CH 3 O· + NO 2 CH 3 O· +O 2 HCHO + HO 2· + NO OH· + NO 2 2{NO 2 + hv (+O 2) NO + O 3} CH 4 + 4 O 2 + 2 hv HCHO + 2 O 3 + H 2 O • HCHO will also undergo further reaction HCHO + hv H 2 + CO H· + HCO HCHO + OH HCO + H 2 O HCO + O 2 HO 2· + CO H· + O 2 HO 2·

Cycle limiting reactions OH· + NO 2 HNO 3 HO 2· + HO 2· H 2 O 2 but also HO 2· + CH 3 O 2· CH 3 OOH + O 2 • methyl hydroperoxide (CH 3 OOH) – can photolyze or react with OH with a lifetime of ~ 2 days • return radicals to system • important source of radicals in upper tropical troposphere – moderately soluble and can be removed from atmosphere by wet or dry deposition • loss of radicals

Conceptually • photolysis of ozone most significant source of OH • atmospheric oxidation of hydrocarbons initiated by OH radical – production of peroxy radicals (HO 2, RO 2) which interact with O 3 -NO-NO 2 cycle to photo-chemically produce ozone – produce carbonyl compounds (aldehydes and ketones) which undergo further oxidation – recycling of OH • termination by formation of nitric acid (OH + NO 2 HNO 3) or peroxides (H 2 O 2, ROOH)

Nitrogen species • NOx (NO + NO 2) plays a critical role in the atmospheric oxidation of hydrocarbons • short chemical lifetime – from ~ 6 hours in PBL to several days to a week in the upper troposphere • large variations in concentration – from 10 s ppbv in urban areas to 10 s pptv in remote regions (UT and remote MBL) • gives rise to different chemical regimes

Regional Ozone perspective - O 3 production • More accurate to talk of NOx/VOC ratio • VOC - volatile organic carbon • High NOx/VOC environments – OH reaction with NO 2 dominates – NO-NO 2 cycling inefficient compared with NOx loss – only found in urban areas • Low NOx/VOC environments – high peroxy radical concentrations – peroxy radical self-reactions become important sink for radicals • production of H 2 O 2 and ROOH

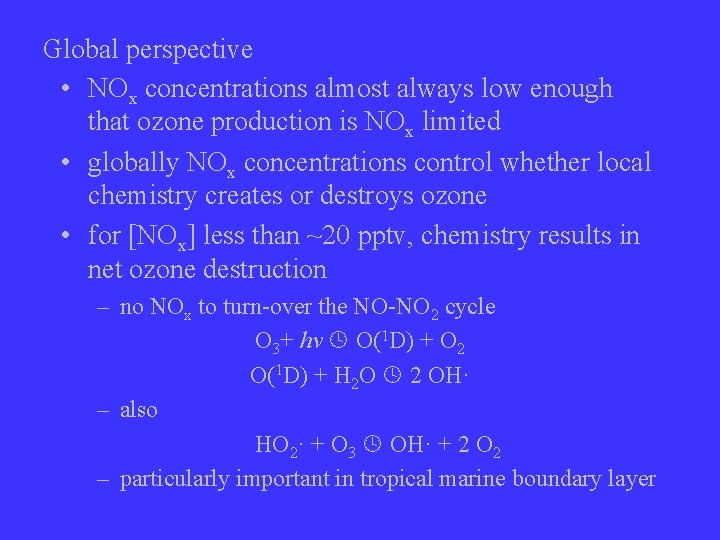
Global perspective • NOx concentrations almost always low enough that ozone production is NOx limited • globally NOx concentrations control whether local chemistry creates or destroys ozone • for [NOx] less than ~20 pptv, chemistry results in net ozone destruction – no NOx to turn-over the NO-NO 2 cycle O 3+ hv O(1 D) + O 2 O(1 D) + H 2 O 2 OH· – also HO 2· + O 3 OH· + 2 O 2 – particularly important in tropical marine boundary layer

Other nitrogen species • Peroxyacyl nitrates (PANs) – most important being peroxyacetyl nitrate • CH 3 C(O)OONO 2 – formed from oxidation of acetaldehyde CH 3 CHO + OH· (+ O 2) CH 3 C(O)O 2 + H 2 O CH 3 C(O)O 2 + NO 2 + M CH 3 C(O)O 2 NO 2 + M – decomposition is strongly temperature dependent • from 30 minutes at 298 K near the surface to several months under upper tropospheric conditions • NOx exported from boundary layer to remote troposphere in the form of PAN – observations show PAN is dominant NOy compound in northern hemisphere spring troposphere • insoluble

Other nitrogen species • N 2 O 5 – formed by NO 2 + O 3 NO 3 + O 2 NO 2 + NO 3 N 2 O 5 – most important is what happens to N 2 O 5 + H 2 O(s) 2 HNO 3 – during daylight fast photolysis of NO 3 limits production of N 2 O 5: NO 3 + hv NO 2 + O

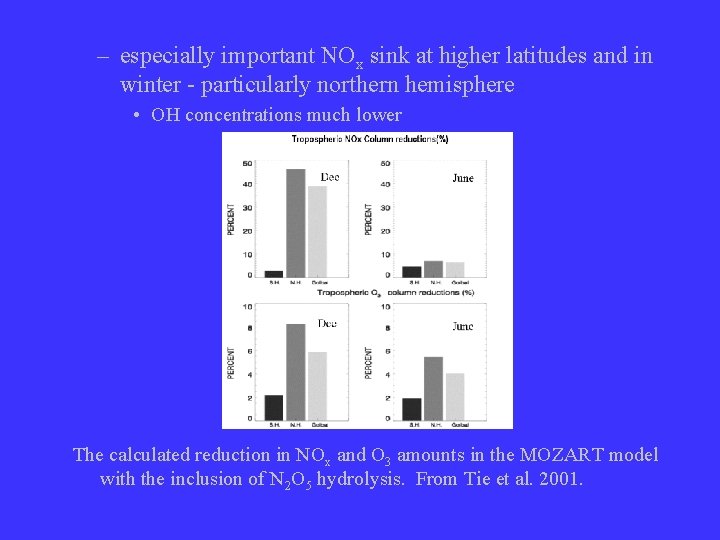
– especially important NOx sink at higher latitudes and in winter - particularly northern hemisphere • OH concentrations much lower The calculated reduction in NOx and O 3 amounts in the MOZART model with the inclusion of N 2 O 5 hydrolysis. From Tie et al. 2001.

NOx Sources Estimates of annual global NOx emissions for the early 1990 s. Units of Tg-N/year. • Biomass burning includes savannah burning, tropical deforestation, temperate wildfires and agricultural waste burning • Soil emission – enhanced by application of fertilizers – largest uncertainty is in estimates of canopy transmission • Lightning – models use ~5. 0 Tg-N/yr – scaling up from observations suggest 20 Tg-N/yr

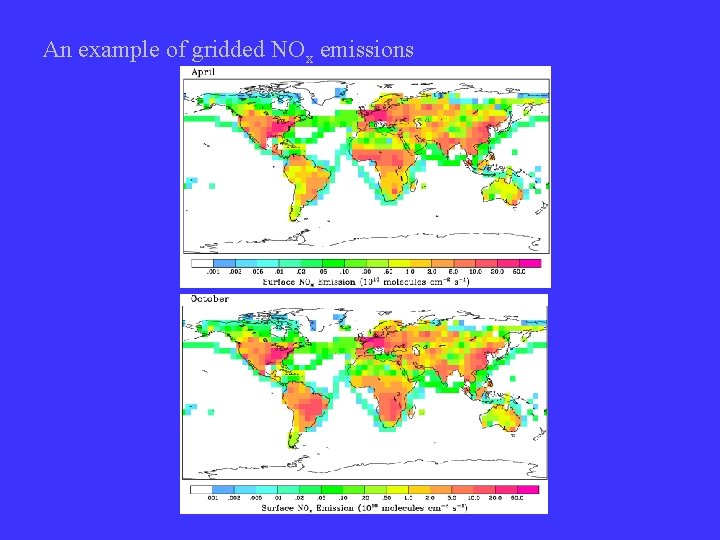
An example of gridded NOx emissions

Impacts of NOx emission • by mass, most NOx is emitted at the surface • chemical impacts of NOx very non-linear – limited impact in the continental PBL • high OH and high NO 2/NO ratio near surface result in a short photo-chemical lifetime • NOx concentrations are already substantial – per molecule, impact of NOx much greater in free troposphere • venting to the free troposphere important • emissions that occur in free troposphere – aircraft, lightning

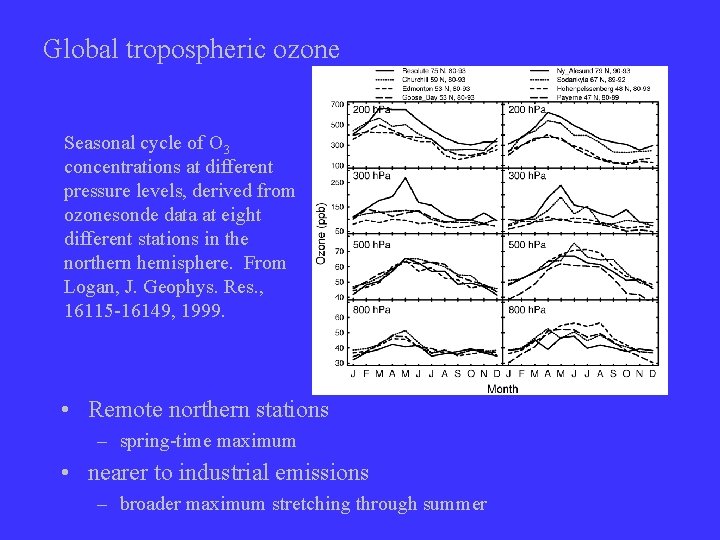
Global tropospheric ozone Seasonal cycle of O 3 concentrations at different pressure levels, derived from ozonesonde data at eight different stations in the northern hemisphere. From Logan, J. Geophys. Res. , 16115 -16149, 1999. • Remote northern stations – spring-time maximum • nearer to industrial emissions – broader maximum stretching through summer

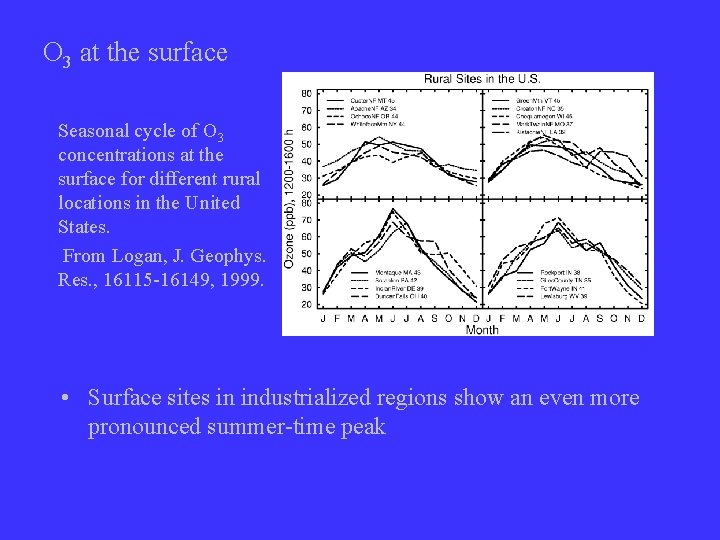
O 3 at the surface Seasonal cycle of O 3 concentrations at the surface for different rural locations in the United States. From Logan, J. Geophys. Res. , 16115 -16149, 1999. • Surface sites in industrialized regions show an even more pronounced summer-time peak

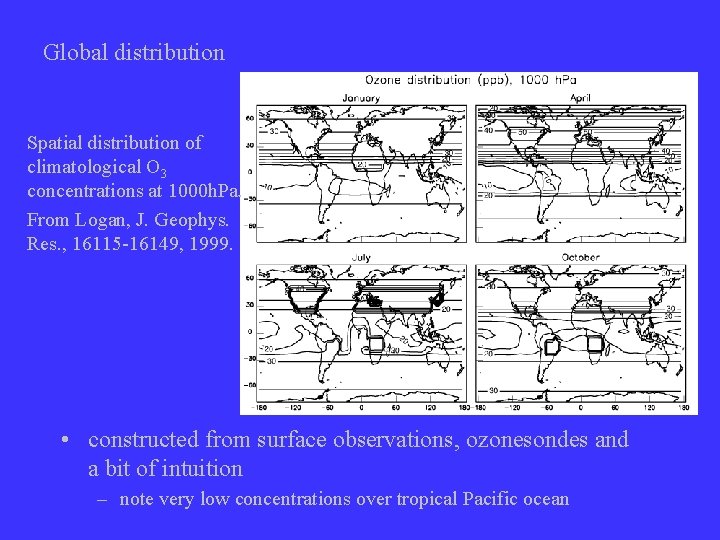
Global distribution Spatial distribution of climatological O 3 concentrations at 1000 h. Pa. From Logan, J. Geophys. Res. , 16115 -16149, 1999. • constructed from surface observations, ozonesondes and a bit of intuition – note very low concentrations over tropical Pacific ocean

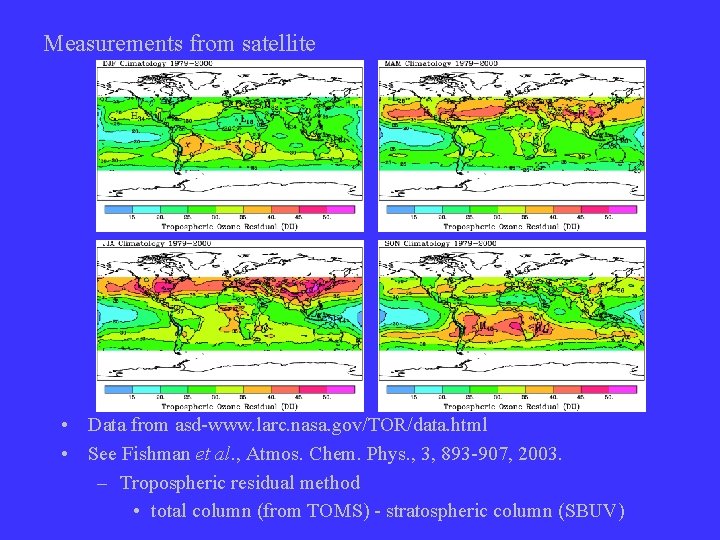
Measurements from satellite • Data from asd-www. larc. nasa. gov/TOR/data. html • See Fishman et al. , Atmos. Chem. Phys. , 3, 893 -907, 2003. – Tropospheric residual method • total column (from TOMS) - stratospheric column (SBUV)

Tropospheric ozone budget • derived from models – a typical budget for present-day conditions: From Lelieveld and Dentener, J. Geophys. Res. , 3531 -3551, 105, 2000

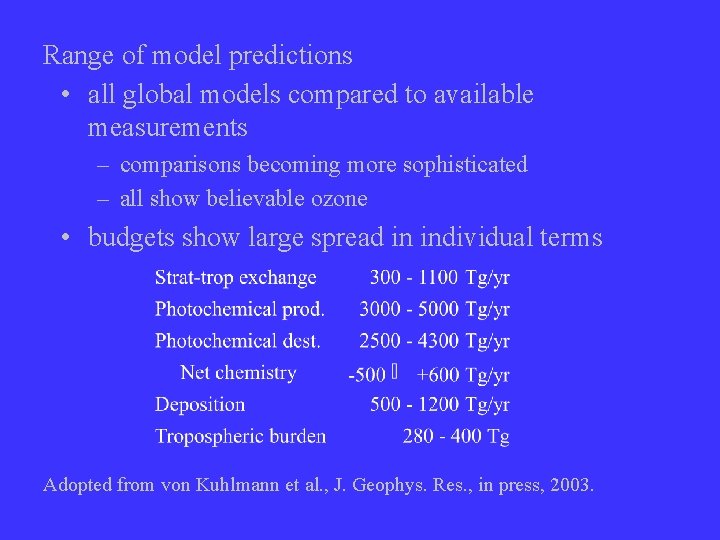
Range of model predictions • all global models compared to available measurements – comparisons becoming more sophisticated – all show believable ozone • budgets show large spread in individual terms Adopted from von Kuhlmann et al. , J. Geophys. Res. , in press, 2003.

Future concerns • How much have emissions of precursors perturbed ozone already? – Ozone is reactive • no ice-core records – some re-constructed records • Montsouris measurements suggested surface O 3 was ~10 ppbv – other information from model simulations • emissions, particularly biomass burning, hard to quantify • suggest tropospheric ozone burden has increased between 25 and 60% since pre-industrial

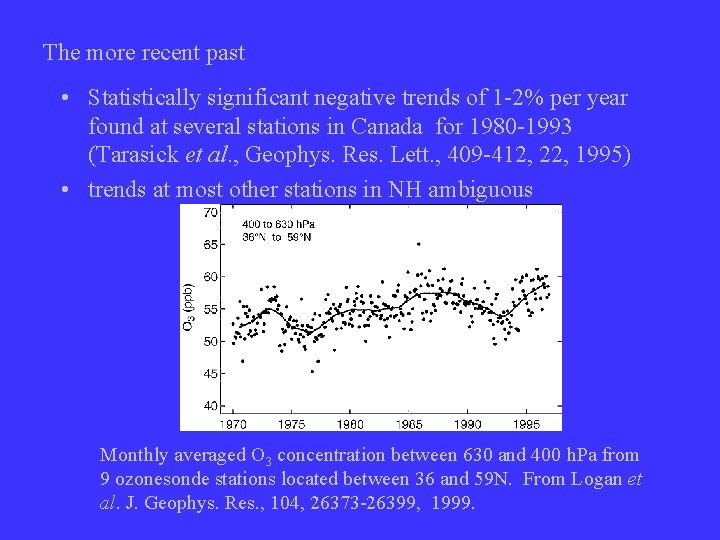
The more recent past • Statistically significant negative trends of 1 -2% per year found at several stations in Canada for 1980 -1993 (Tarasick et al. , Geophys. Res. Lett. , 409 -412, 22, 1995) • trends at most other stations in NH ambiguous Monthly averaged O 3 concentration between 630 and 400 h. Pa from 9 ozonesonde stations located between 36 and 59 N. From Logan et al. J. Geophys. Res. , 104, 26373 -26399, 1999.

IPCC Ox. Comp simulations for 2100 • Emissions for year 2100 were a bit of a ‘worst case’ scenario CH 4 = 4. 3 ppmv; NOx = 110 Tg-N/yr (32. 5) CO = 2500 Tg/yr (1050); VOC = 350 Tg/yr (150) • mid-latitude O 3 increases by 20 -30 ppbv at the surface – puts background O 3 in 60 -70 ppbv range • these models did not include impacts of global warming – increased H 2 O vapour – temperature effects on reaction rates • increasingly coupled models – inclusion of biosphere-atmosphere interactions – lightning

Stability of global OH • OH originates with O 3 – very reactive and very short-lived – recycling critically important • OH is responsible for initiating atmospheric oxidation of hydrocarbons – CH 4 lifetime of ~10 years • are changes in chemical composition of the troposphere affecting average OH?

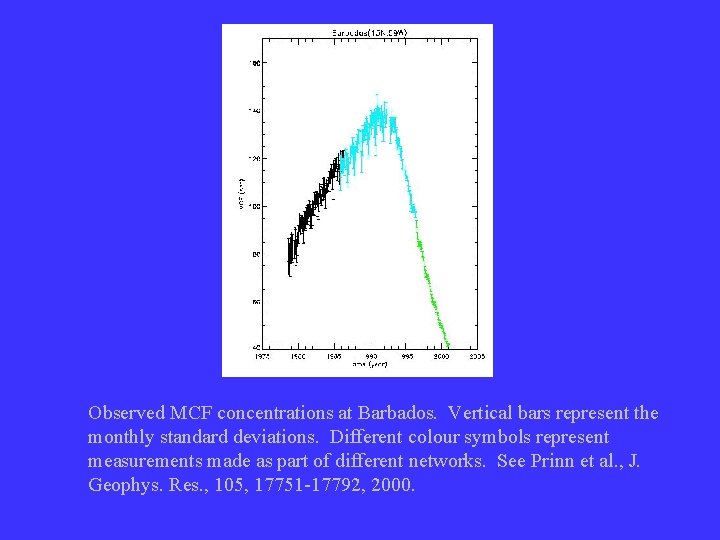
Information from methyl chloroform • CH 3 CCl 3 used as solvent by industry – atmospheric lifetime of 5 -6 years • main loss by reaction with OH • some entered stratosphere and enhanced Cl levels – banned under Montreal protocol • use was to stop in 1996 in developed countries – assuming one knows the sources of MCF, it is possible to calculate an average global OH by fitting to observed decay

Observed MCF concentrations at Barbados. Vertical bars represent the monthly standard deviations. Different colour symbols represent measurements made as part of different networks. See Prinn et al. , J. Geophys. Res. , 105, 17751 -17792, 2000.

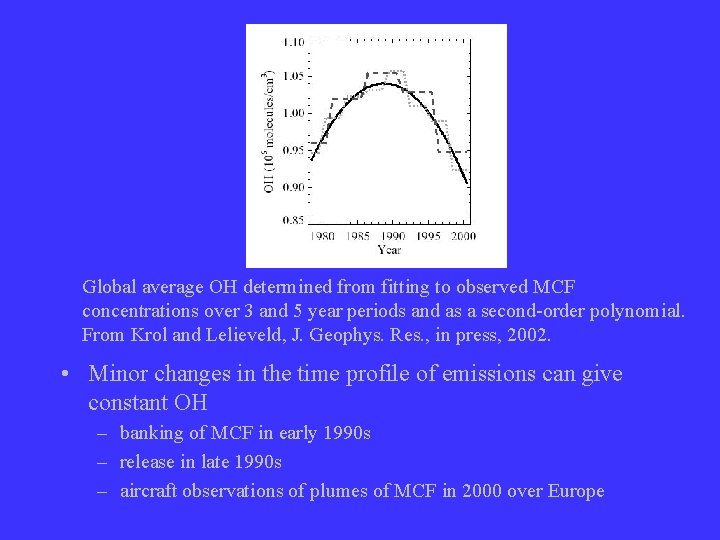
Global average OH determined from fitting to observed MCF concentrations over 3 and 5 year periods and as a second-order polynomial. From Krol and Lelieveld, J. Geophys. Res. , in press, 2002. • Minor changes in the time profile of emissions can give constant OH – banking of MCF in early 1990 s – release in late 1990 s – aircraft observations of plumes of MCF in 2000 over Europe
 Plummer vinson syndrome
Plummer vinson syndrome Joan plummer
Joan plummer Joan plummer
Joan plummer Plummer-vinson syndrome بالعربي
Plummer-vinson syndrome بالعربي Granular bed
Granular bed Hagen poiseuille equation
Hagen poiseuille equation Presbydysphagia
Presbydysphagia Ozone layer definition
Ozone layer definition How do cfcs destroy ozone
How do cfcs destroy ozone Protective ozone layer
Protective ozone layer Ozone composition
Ozone composition Photonic medicine
Photonic medicine Microplasma ozone
Microplasma ozone Protective ozone layer
Protective ozone layer Ozone layer depletion
Ozone layer depletion Ozone layer simple definition
Ozone layer simple definition The ozone blanket blocks
The ozone blanket blocks Ccl2f2 lewis
Ccl2f2 lewis Chemical security awareness training
Chemical security awareness training Ozone depletion effect on humans
Ozone depletion effect on humans O3 lewis structure molecular geometry
O3 lewis structure molecular geometry Methyl nitrite lewis structure
Methyl nitrite lewis structure Ozone layer
Ozone layer Ozone layer depletion introduction
Ozone layer depletion introduction Poisonous
Poisonous Stratospheric ozone depletion
Stratospheric ozone depletion Trou d'ozone
Trou d'ozone How is total ozone distributed over the globe
How is total ozone distributed over the globe Jamoytius
Jamoytius Ozone layer made up of
Ozone layer made up of Protection of ozone layer
Protection of ozone layer Vray sun turbidity
Vray sun turbidity Ozone depletion diagram
Ozone depletion diagram Cause of ozone depletion
Cause of ozone depletion Ozone without borders
Ozone without borders Ozone hole myth
Ozone hole myth Ozone layer levels
Ozone layer levels