Trans Celerate Overview Updated 13 July 2020 Trans










- Slides: 21

Trans. Celerate Overview Updated: 13 July, 2020

Trans. Celerate: A Not-for-Profit Entity Created to Foster Collaboration Our Shared Vision: To improve the health of people around the world by accelerating and simplifying the research and development of innovative new therapies. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 2

Current state of organization 2012 Trans. Celerate Founded 10 5 MEMBER COMPANIE S 2016 Bio. Celerate Founded focus on preclinical research Today 20 MEMBER COMPANIE S 30 + Bayer most recent member INITIAL INITIATIVES BREADTH & DEPTH Over 60 solutions being delivered across 25+ initiatives, across 3 strategic priorities INITIATIVES including 5 pharmacovigilance initiatives ENHANCING INDUSTRY COLLABORATION With an effective and proven governance structure have increased the ease and desire to collaborate FACILITATING FUTURE PLATFORM TRIALS 12+ initiatives deliver solutions that facilitate future platform trials platform to enable data sharing Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 3

The Reach of our Global Membership is Expanding Membership is available to biopharmaceutical research and development organizations that engage in innovative discovery, development and manufacturing of new medicines*. Thousands of people have contributed to the design, development and deployment of Trans. Celerate solutions Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * to be eligible for membership, companies must meet specified eligibility criteria. 4

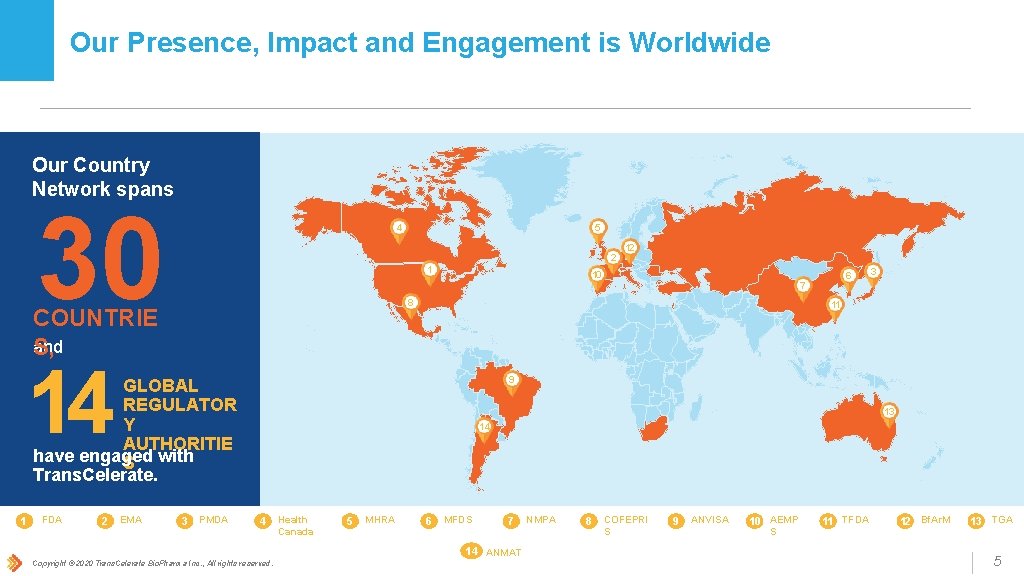
Our Presence, Impact and Engagement is Worldwide Our Country Network spans 30 5 4 2 1 10 14 2 EMA 3 PMDA 3 11 9 GLOBAL REGULATOR Y AUTHORITIE have engaged S with Trans. Celerate. FDA 6 7 8 COUNTRIE and S, 1 12 13 14 4 Health Canada 5 MHRA 6 MFDS 7 14 ANMAT Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. NMPA 8 COFEPRI S 9 ANVISA 10 AEMP S 11 TFDA 12 Bf. Ar. M 13 TGA 5

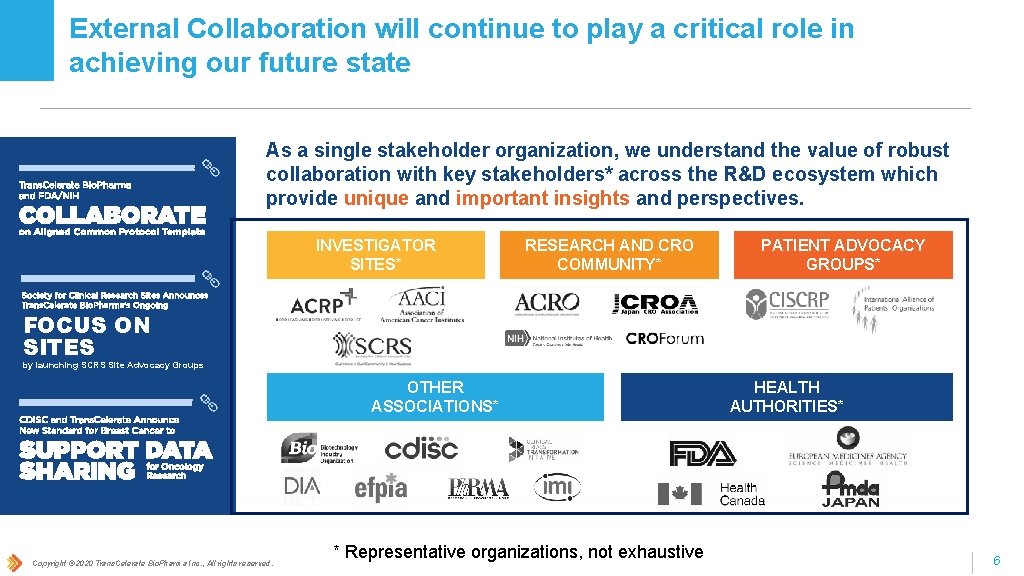
External Collaboration will continue to play a critical role in achieving our future state As a single stakeholder organization, we understand the value of robust collaboration with key stakeholders* across the R&D ecosystem which provide unique and important insights and perspectives. INVESTIGATOR SITES* RESEARCH AND CRO COMMUNITY* PATIENT ADVOCACY GROUPS* FOCUS ON SITES by launching SCRS Site Advocacy Groups OTHER ASSOCIATIONS* Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Representative organizations, not exhaustive HEALTH AUTHORITIES* 6

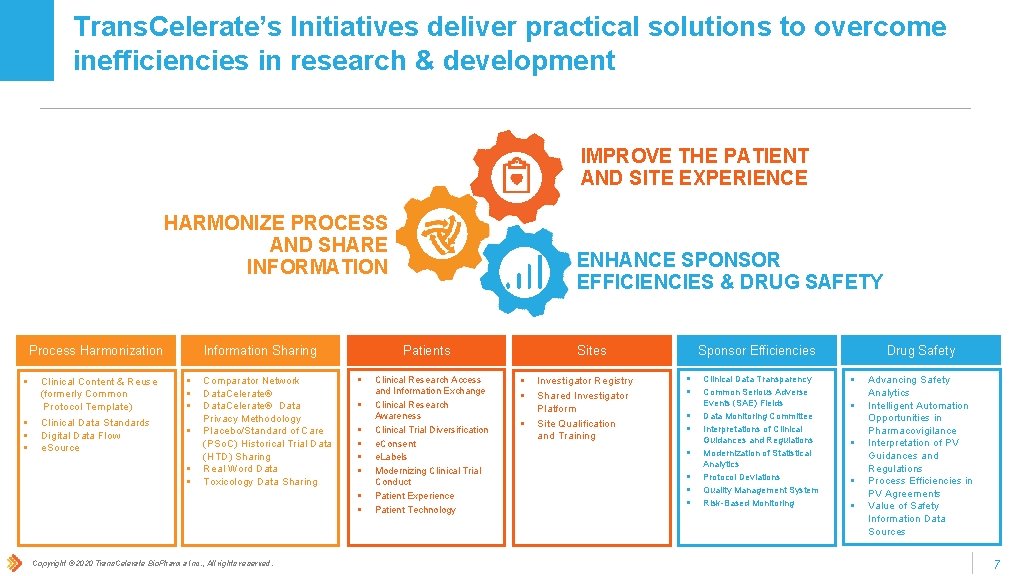
Trans. Celerate’s Initiatives deliver practical solutions to overcome inefficiencies in research & development IMPROVE THE PATIENT AND SITE EXPERIENCE HARMONIZE PROCESS AND SHARE INFORMATION Process Harmonization § § Information Sharing Clinical Content & Reuse (formerly Common Protocol Template) § § § Clinical Data Standards Digital Data Flow e. Source § § § Comparator Network Data. Celerate® Data Privacy Methodology Placebo/Standard of Care (PSo. C) Historical Trial Data (HTD) Sharing Real Word Data Toxicology Data Sharing Patients § § § § Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. ENHANCE SPONSOR EFFICIENCIES & DRUG SAFETY Clinical Research Access and Information Exchange Clinical Research Awareness Clinical Trial Diversification e. Consent e. Labels Modernizing Clinical Trial Conduct Patient Experience Patient Technology Sites § § § Investigator Registry Shared Investigator Platform Site Qualification and Training Drug Safety Sponsor Efficiencies § § § § Clinical Data Transparency Common Serious Adverse Events (SAE) Fields Data Monitoring Committee Interpretations of Clinical Guidances and Regulations Modernization of Statistical Analytics Protocol Deviations Quality Management System Risk-Based Monitoring § § § Advancing Safety Analytics Intelligent Automation Opportunities in Pharmacovigilance Interpretation of PV Guidances and Regulations Process Efficiencies in PV Agreements Value of Safety Information Data Sources 7

Our Work in Preclinical Development: Bio. Celerate, a subsidiary of Trans. Celerate Bio. Pharma, focuses on the identification and development of pragmatic and tangible solutions to improve efficiencies in preclinical research. Leadership and Governance Structure: Bio. Celerate has been established as a separate legal subsidiary of Trans. Celerate with separate funding and support. Toxicology and Background Control Data Sharing Initiative The first Initiative, Toxicology Data Sharing, is working to help close critical gaps between patient response and preclinical toxicology findings. Nonclinical Study Optimization The initiative is focused on working with key stakeholders to implement common best practices such as harmonization of SEND data sets and authoring of protocols and reports. Membership in Trans. Celerate is a prerequisite for Bio. Celerate membership. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 8

Our Work in Data Sharing: Data. Celerate will be used to aggregate and analyze preclinical and clinical information* to improve drug development efficiency and bring new medicines to patients faster. Toxicology & Background Control Data Placebo Standard of Care Data May, 2018 2019 Enables participating companies to make datadriven decisions on compound progression based on an increased understanding of ontarget and off-target toxicity. Enables participating Trans. Celerate members to collect and share anonymized clinical data historically gathered in the placebo and standardof-care arms of clinical trials among participating member companies. *Additional data types currently under assessment Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 9

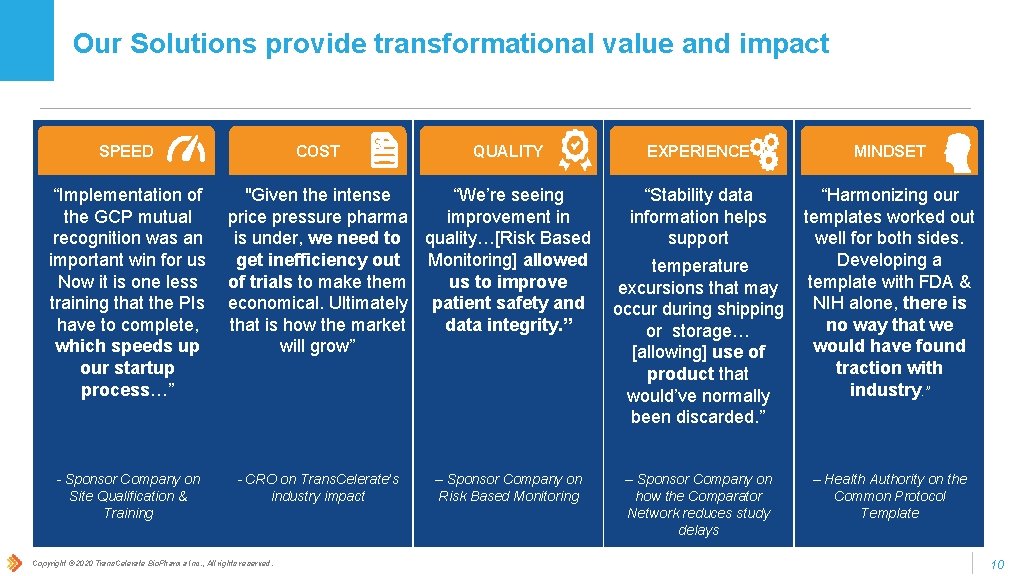
Our Solutions provide transformational value and impact SPEED “Implementation of the GCP mutual recognition was an important win for us Now it is one less training that the PIs have to complete, which speeds up our startup process…” - Sponsor Company on Site Qualification & Training COST QUALITY “We’re seeing "Given the intense improvement in price pressure pharma is under, we need to quality…[Risk Based Monitoring] allowed get inefficiency out us to improve of trials to make them economical. Ultimately patient safety and data integrity. ” that is how the market will grow” - CRO on Trans. Celerate’s industry impact Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. – Sponsor Company on Risk Based Monitoring EXPERIENCE MINDSET “Stability data information helps support “Harmonizing our templates worked out well for both sides. Developing a template with FDA & NIH alone, there is no way that we would have found traction with industry. ” temperature excursions that may occur during shipping or storage… [allowing] use of product that would’ve normally been discarded. ” – Sponsor Company on how the Comparator Network reduces study delays – Health Authority on the Common Protocol Template 10

Visit us, for more information: www. Trans. Celerate. Bio. Pharma. Inc. com Watch our “About Us” Video Sign up for our Newsletter, Accelerate to Innovate @Trans. Celerate Bio. Pharma Inc.

Appendix

Trans. Celerate Tools and Resources VIDEOS About Us Patients Sponsors Sites Info Sharing & Harmonization e. Consent e. Labels Common Protocol Template Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. INITIATIVE ASSETS Clinical Trial Registry of the Future e. Book RBM Interactive Guide ABOUT TRANSCELERATE Brochure Accelerate to Innovate The Pulse on Progress Common Protocol Template Assets e. Consent Implementation Guidance e. Labels Design & Delivery Toolkit 13

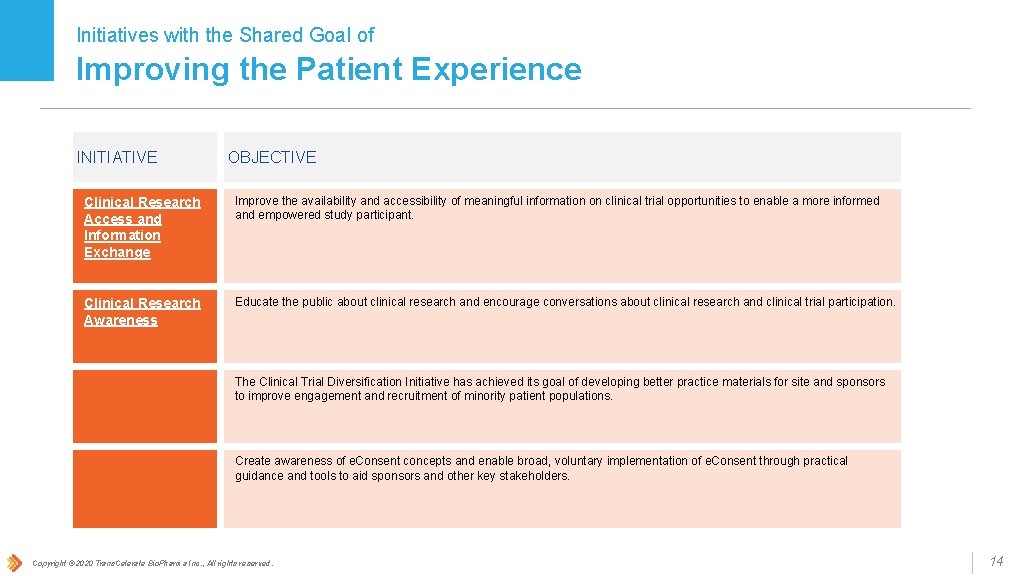
Initiatives with the Shared Goal of Improving the Patient Experience INITIATIVE OBJECTIVE Clinical Research Access and Information Exchange Improve the availability and accessibility of meaningful information on clinical trial opportunities to enable a more informed and empowered study participant. Clinical Research Awareness Educate the public about clinical research and encourage conversations about clinical research and clinical trial participation. Clinical Trial Diversification The Clinical Trial Diversification Initiative has achieved its goal of developing better practice materials for site and sponsors to improve engagement and recruitment of minority patient populations. e. Consent Create awareness of e. Consent concepts and enable broad, voluntary implementation of e. Consent through practical guidance and tools to aid sponsors and other key stakeholders. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 14

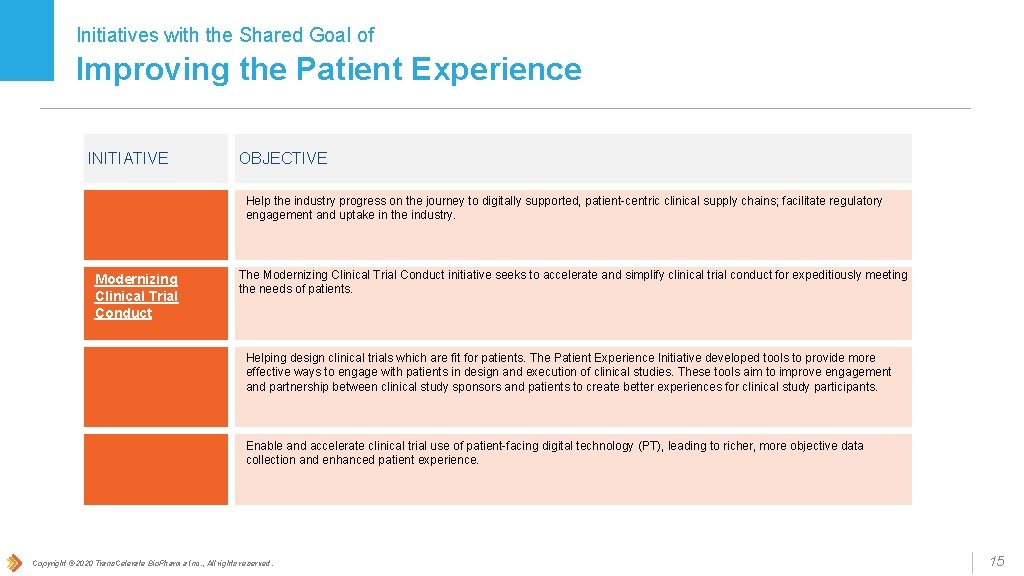
Initiatives with the Shared Goal of Improving the Patient Experience INITIATIVE e. Labels Modernizing Clinical Trial Conduct OBJECTIVE Help the industry progress on the journey to digitally supported, patient-centric clinical supply chains; facilitate regulatory engagement and uptake in the industry. The Modernizing Clinical Trial Conduct initiative seeks to accelerate and simplify clinical trial conduct for expeditiously meeting the needs of patients. Patient Experience Helping design clinical trials which are fit for patients. The Patient Experience Initiative developed tools to provide more effective ways to engage with patients in design and execution of clinical studies. These tools aim to improve engagement and partnership between clinical study sponsors and patients to create better experiences for clinical study participants. Patient Technology Enable and accelerate clinical trial use of patient-facing digital technology (PT), leading to richer, more objective data collection and enhanced patient experience. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 15

Initiatives with the Shared Goal of Improving the Site Experience INITIATIVE OBJECTIVE Investigator Registry Accelerates identification and recruitment of sites via a repository of consenting investigator contact and enrollment data and enhances the Shared Investigator Platform (SIP). Shared Investigator Platform Facilitates interaction between investigators and multiple clinical trial sponsors, enabling study planning, study start-up, and study conduct activities while reducing administrative burden on sites. Site Qualification and Training Enhance and simplify clinical trial site qualification and training processes and reduce the administrative burden on sites. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 16

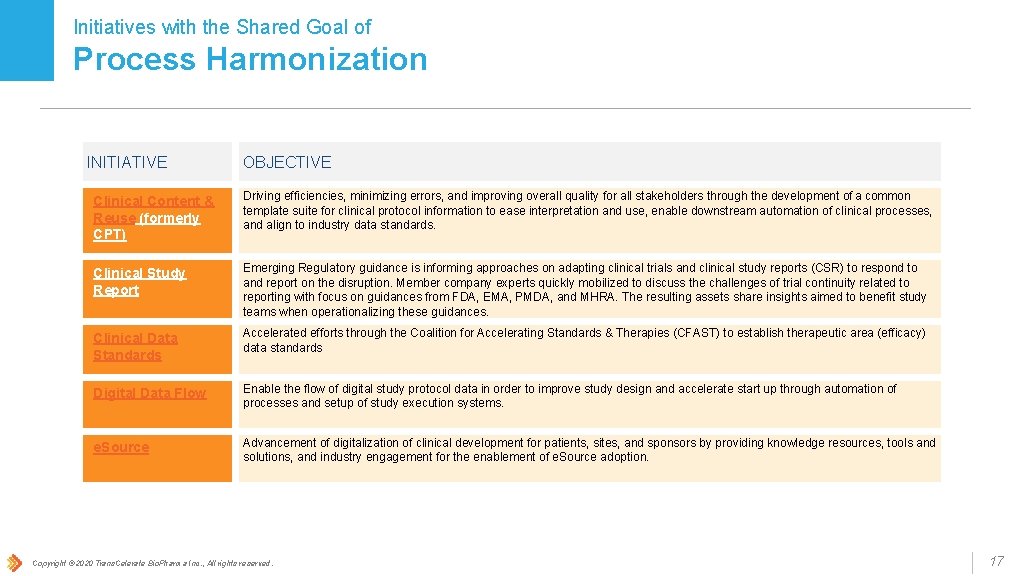
Initiatives with the Shared Goal of Process Harmonization INITIATIVE OBJECTIVE Clinical Content & Reuse (formerly CPT) Driving efficiencies, minimizing errors, and improving overall quality for all stakeholders through the development of a common template suite for clinical protocol information to ease interpretation and use, enable downstream automation of clinical processes, and align to industry data standards. Clinical Study Report Emerging Regulatory guidance is informing approaches on adapting clinical trials and clinical study reports (CSR) to respond to and report on the disruption. Member company experts quickly mobilized to discuss the challenges of trial continuity related to reporting with focus on guidances from FDA, EMA, PMDA, and MHRA. The resulting assets share insights aimed to benefit study teams when operationalizing these guidances. Clinical Data Standards Accelerated efforts through the Coalition for Accelerating Standards & Therapies (CFAST) to establish therapeutic area (efficacy) data standards Digital Data Flow Enable the flow of digital study protocol data in order to improve study design and accelerate start up through automation of processes and setup of study execution systems. e. Source Advancement of digitalization of clinical development for patients, sites, and sponsors by providing knowledge resources, tools and solutions, and industry engagement for the enablement of e. Source adoption. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 17

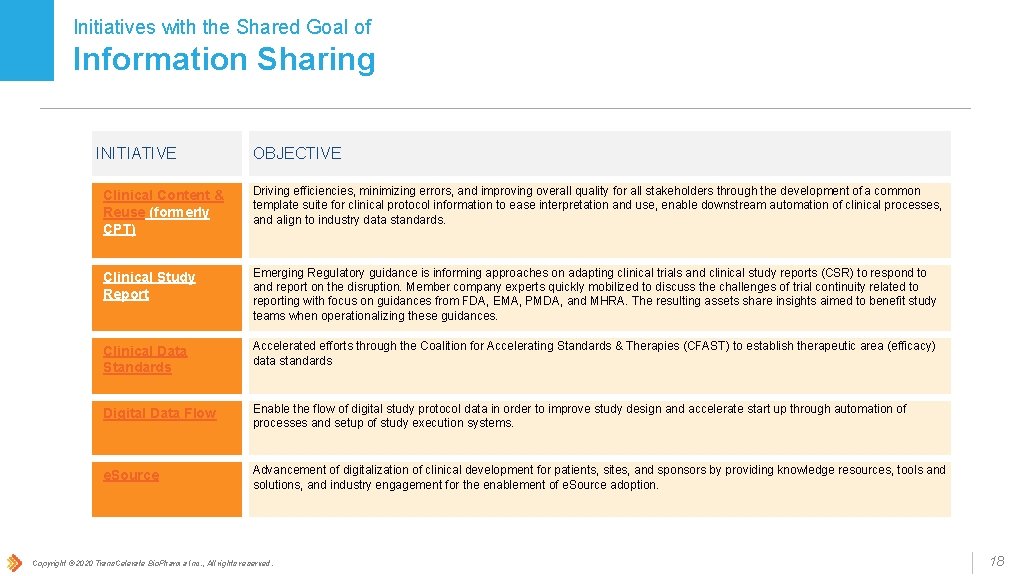
Initiatives with the Shared Goal of Information Sharing INITIATIVE OBJECTIVE Clinical Content & Reuse (formerly CPT) Driving efficiencies, minimizing errors, and improving overall quality for all stakeholders through the development of a common template suite for clinical protocol information to ease interpretation and use, enable downstream automation of clinical processes, and align to industry data standards. Clinical Study Report Emerging Regulatory guidance is informing approaches on adapting clinical trials and clinical study reports (CSR) to respond to and report on the disruption. Member company experts quickly mobilized to discuss the challenges of trial continuity related to reporting with focus on guidances from FDA, EMA, PMDA, and MHRA. The resulting assets share insights aimed to benefit study teams when operationalizing these guidances. Clinical Data Standards Accelerated efforts through the Coalition for Accelerating Standards & Therapies (CFAST) to establish therapeutic area (efficacy) data standards Digital Data Flow Enable the flow of digital study protocol data in order to improve study design and accelerate start up through automation of processes and setup of study execution systems. e. Source Advancement of digitalization of clinical development for patients, sites, and sponsors by providing knowledge resources, tools and solutions, and industry engagement for the enablement of e. Source adoption. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 18

Initiatives with the Shared Goal of Enhancing Sponsor Efficiencies INITIATIVE OBJECTIVE Clinical Data Transparency Develop model approaches for redacting personal data found in clinical study reports & for the anonymization/de-identification of patient level data shared with the broader healthcare community. Common Serious Adverse Events (SAE) Fields Identify and validate a common set of SAE reporting fields. The output of this initiative will be transitioned to CDISC for development into an industry standard. Data Monitoring Committee Identify opportunities to increase the pool of DMC candidates and provide approaches for newly identified individuals to gain experience through mentoring relationships. Interpretation of Guidances & Regulations The Interpretation of Guidances & Regulations Initiative will share expertise to more efficiently and effectively meet the intent of ambiguous regulatory requirements and guidances in both the pharmacovigilance and clinical areas. Modernization of Statistical Analytics Develop a foundational framework which instills confidence in the use of technologies to potentially enable a more modern statistical analytical environment. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 19

Initiatives with the Shared Goal of Enhancing Sponsor Efficiencies INITIATIVE OBJECTIVE Protocol Deviations Working with relevant Health Authorities to create a Protocol Deviation (PD) Management Toolkit designed to help sponsors conducting clinical research improve PD classification, collection, analysis, and reporting processes. Quality Management System In the absence of consistent regulatory guidance, published an integrated, proactive, flexible conceptual framework, and associated operational tools, which clinical development organizations may use to systematically define quality objectives linked to their broader strategic goals. Risk-Based Monitoring Published model guidelines and associated operational tools for targeted, risk-based clinical trial monitoring, ultimately aiming to improve patient safety and data quality. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 20

Initiatives with the Shared Goal of Drug Safety INITIATIVE OBJECTIVE Advancing Safety Analytics Develop best practices and guidance around the application of interrogative methods towards various safety data sources. Intelligent Automation Opportunities in Pharmacovigilance This initiative focuses on identifying how intelligent automation technologies can be used to better support execution of Pharmacovigilance activities/processes. Interpretation of PV Guidances & Regulations Share expertise to more efficiently and effectively meet the intent of ambiguous Pharmacovigilance regulations/guidances and operational expectations around the world. The initiative will also take opportunities to raise Health Authority awareness of the real-life impact of ambiguous regulations/guidelines. Pharmacovigilance Agreements (launched April 2020) Addressing inefficiencies relating to PV agreements which wastes resources and contributes to non-value adding work due to confusion about contractual terms and definitions, lengthy negotiation periods and lack of widely accepted contract structure. Value of Safety Information Data Sources Identify sources of safety information for single high value valid cases and develop a proposed method for management of lower informative cases to present to regulators. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 21
 Trans celerate
Trans celerate Trans celerate
Trans celerate Tender mean
Tender mean The symbol tsfa in alu operations include
The symbol tsfa in alu operations include Performance monitoring and coaching form 2021
Performance monitoring and coaching form 2021 Monday 13th july
Monday 13th july May 1775
May 1775 July 16 1776
July 16 1776 July 2 1937 amelia earhart
July 2 1937 amelia earhart Harris burdick pictures uninvited guest
Harris burdick pictures uninvited guest Ctdssmap payment schedule july 2021
Ctdssmap payment schedule july 2021 Catawba indian nation bingo
Catawba indian nation bingo 2003 july 17
2003 july 17 Malaga in july
Malaga in july On july 18 2001 a train carrying hazardous chemicals
On july 18 2001 a train carrying hazardous chemicals Miss cuba receives an invitation
Miss cuba receives an invitation I am silver and exact i have no preconceptions
I am silver and exact i have no preconceptions June 22 to july 22
June 22 to july 22 July 1-4 1863
July 1-4 1863 Sergei korolev
Sergei korolev July 30 2009 nasa
July 30 2009 nasa Why are leaf yeasts more plentiful in july
Why are leaf yeasts more plentiful in july