Ticagrelor With Asp Irin or ALone In Hi




![Prespecified Ischemic Endpoints (PP Cohort) 5% HR [95%CI]: 0. 97 [0. 76 - 1. Prespecified Ischemic Endpoints (PP Cohort) 5% HR [95%CI]: 0. 97 [0. 76 - 1.](https://slidetodoc.com/presentation_image_h2/6498e48bdbb3b783eae0b646eb1df027/image-26.jpg)





- Slides: 38

Ticagrelor With Asp. Irin or ALone In Hi. GH-Risk Patients After Coronary In. Tervention Roxana Mehran, MD on behalf of the TWILIGHT Investigators Icahn School of Medicine at Mount Sinai, New York, NY Clinical. Trials. gov Number: NCT 02270242

Declaration of Interest The TWILIGHT Trial Sponsoring organization: Icahn School of Medicine at Mount Sinai, NY Funded by Astra. Zeneca Coordinated by Icahn School of Medicine at Mount Sinai, NY

Disclosures Affiliation/Financial Relationship Company Consultant/ Exec committee/Advisory board/personal fees Abbott Laboratories, Boston Scientific, Medscape, Siemens Medical Solutions, Phillips (Spectranetics), PLx Pharma, Roivant Sciences Inc, Volcano Corporation, Sanofi, Janssen, Research Funding to Institution Abbott Laboratories, Astra Zeneca, Bayer, Beth Israel Deaconess, BMS, CSL Behring, DSI, Medtronic, Boston Scientific, Novartis, Orbus. Neich Equity, <1% Claret Medical, Elixir Medical DSMB membership paid to the institution Watermark Research Partners

Background • Balancing ischemic and bleeding complications post PCI is an important dilemma for clinicians. 1 -3 • Addressing the clinical imperatives of lowering bleeding while preserving ischemic benefit requires therapeutic strategies that decouple thrombotic from hemorrhagic risk. • Reducing the duration of aspirin after PCI may allow for more prolonged use of potent P 2 Y 12 inhibitors while avoiding aspirin-related bleeding risk. 4 1. Baber et al. JACC Cardiovasc Interv 2016; 9: 1349 -57. 2. Genereux et al. J Am Coll Cardiol 2015; 66: 1036 -45. 3. Valgimigli et al. Eur Heart J 2017; 38: 804 -10. 4. Capodanno et al. Nat Rev Cardiol 2018; 15: 480 -96.

Trial Hypothesis In patients undergoing PCI who are at high risk for ischemic or hemorrhagic complications and who have completed a 3 -month course of dual antiplatelet therapy with ticagrelor plus aspirin, continued treatment with ticagrelor monotherapy would be superior to ticagrelor plus aspirin with respect to clinically relevant bleeding and would not lead to ischemic harm.

Trial Objectives Primary Objective: To determine the impact of SAPT (ticagrelor monotherapy) versus DAPT (ticagrelor plus aspirin) for 12 months in reducing clinically relevant bleeding (BARC 2, 3 or 5) among high-risk patients who have undergone successful PCI. Secondary Objective: To determine the impact of SAPT (ticagrelor monotherapy) versus DAPT (ticagrelor plus aspirin) for 12 months on major ischemic adverse events (all-cause death, non-fatal MI or stroke) among high-risk patients who have undergone successful PCI.

Methods TWILIGHT was a randomized, double-blinded, placebo-controlled trial conducted in 187 sites across 11 countries. From July 2015 - December 2017, patients undergoing successful PCI with at least 1 locally-approved DES whom the treating clinician intended to discharge on ticagrelor plus aspirin were eligible to participate. Trial inclusion required the presence of at least 1 additional clinical and angiographic feature associated with a high risk of ischemic or bleeding events.

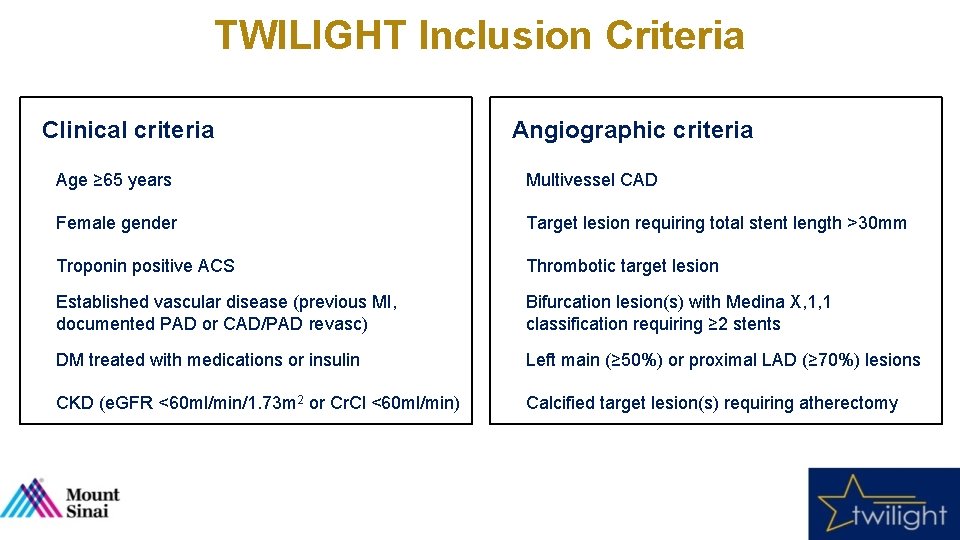
TWILIGHT Inclusion Criteria Clinical criteria Angiographic criteria Age ≥ 65 years Multivessel CAD Female gender Target lesion requiring total stent length >30 mm Troponin positive ACS Thrombotic target lesion Established vascular disease (previous MI, documented PAD or CAD/PAD revasc) Bifurcation lesion(s) with Medina X, 1, 1 classification requiring ≥ 2 stents DM treated with medications or insulin Left main (≥ 50%) or proximal LAD (≥ 70%) lesions CKD (e. GFR <60 ml/min/1. 73 m 2 or Cr. Cl <60 ml/min) Calcified target lesion(s) requiring atherectomy

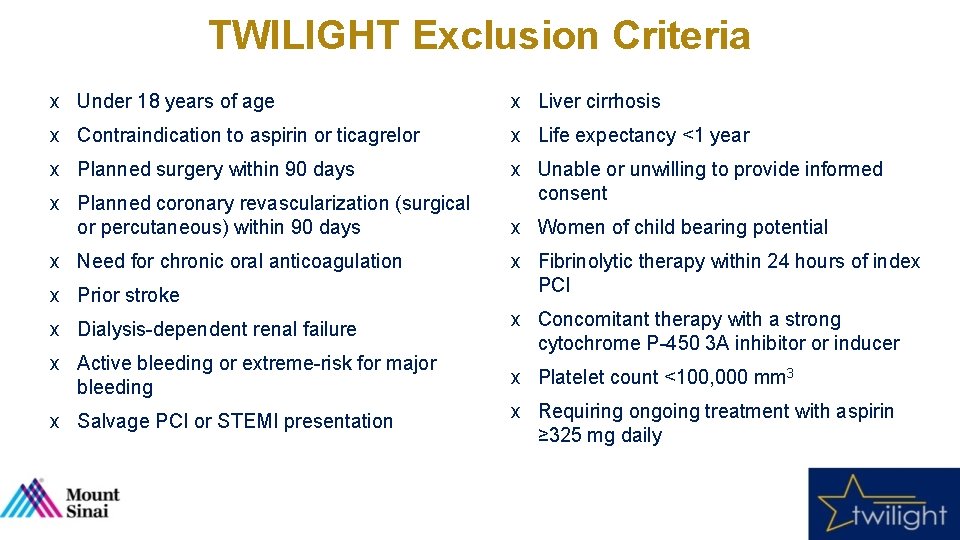
TWILIGHT Exclusion Criteria x Under 18 years of age x Liver cirrhosis x Contraindication to aspirin or ticagrelor x Life expectancy <1 year x Planned surgery within 90 days x Unable or unwilling to provide informed consent x Planned coronary revascularization (surgical or percutaneous) within 90 days x Need for chronic oral anticoagulation x Prior stroke x Dialysis-dependent renal failure x Active bleeding or extreme-risk for major bleeding x Salvage PCI or STEMI presentation x Women of child bearing potential x Fibrinolytic therapy within 24 hours of index PCI x Concomitant therapy with a strong cytochrome P-450 3 A inhibitor or inducer x Platelet count <100, 000 mm 3 x Requiring ongoing treatment with aspirin ≥ 325 mg daily

Study Organization Executive Committee Roxana Mehran Usman Baber Dominick J. Angiolillo David Cohen Data Coordinating Center for Interventional Cardiovascular Research and Clinical Trials at Mount Sinai, New York Trial Leadership Country Leaders Austria Kurt Huber Israel Giora Weisz, Ran Kornowski UK Vijay Kunadian, Keith Oldroyd China Han Yaling George D. Dangas Roxana Mehran Global PI India Upendra Kaul Shamir Mehta Usman Baber Chair, Clinical Coordinating Center Germany Berhard Witzenbichler C. Michael Gibson Theresa Franklin-Bond Pharmacovigilance & Safety Canada Vladimir Dzavik Adnan Kastrati Jerusa Altema Project Manager Poland Robert Gil, Dariuz Dudek Mitchel Krucoff Samantha Sartori Statistician Italy Gennaro Sardella E. Magnus Ohman CEC Spain Javier Escaned Philippe Gabriel Steg Jin Y. Cha (Manager) Steering Committee Steven O. Marx (Chair) Bruce Darrow Douglas Di. Stefano Newsha Ghodsi Jose Meller Nicola Corvaia S. Chiu Wong Paul Gurbel Christian Hamm Timothy D. Henry David J. Moliterno Samin K. Sharma Stroke adjudication: Jesse Weinberger David Kaufman Mark Milstein Sponsor: Icahn School of Medicine at Mount Sinai Funding: Astra. Zeneca DSMB Bernard J. Gersh (Chair) Spencer King Stuart Pocock David Faxon James Tcheng Tim Collier (Statistician)

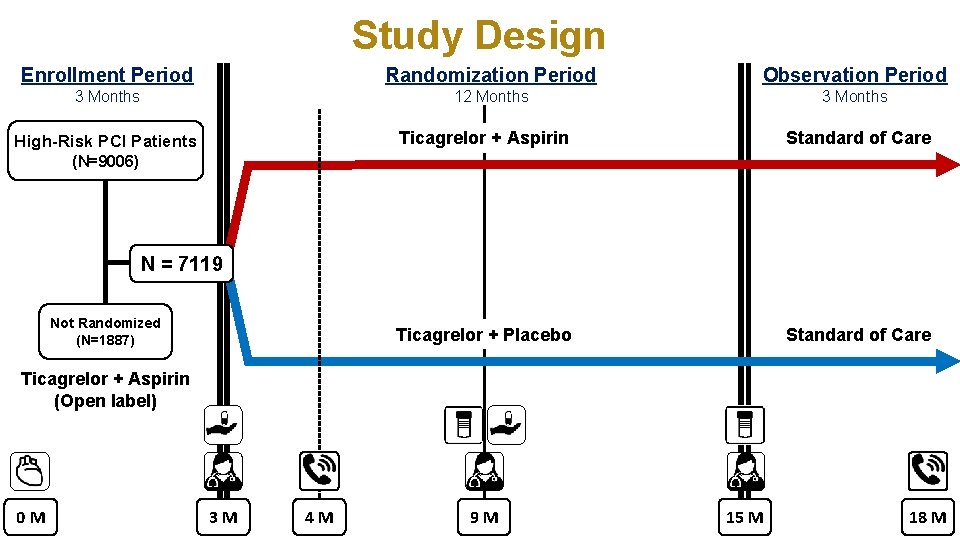
Study Design Enrollment Period Randomization Period Observation Period 3 Months 12 Months 3 Months High-Risk PCI Patients Ticagrelor + Aspirin Standard of Care Ticagrelor + Placebo Standard of Care (N=9006) N = 7119 Not Randomized (N=1887) Ticagrelor + Aspirin (Open label) 0 M 3 M 4 M 9 M 15 M 18 M

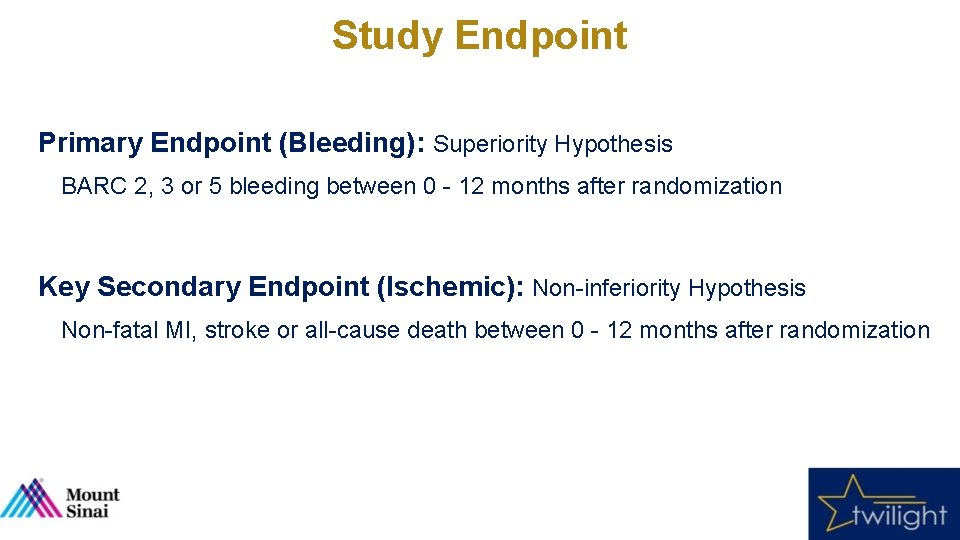
Study Endpoint Primary Endpoint (Bleeding): Superiority Hypothesis BARC 2, 3 or 5 bleeding between 0 - 12 months after randomization Key Secondary Endpoint (Ischemic): Non-inferiority Hypothesis Non-fatal MI, stroke or all-cause death between 0 - 12 months after randomization

Sample Size and Power Calculations Assuming a one-year rate of BARC 2, 3 or 5 bleeding of 4. 5% among patients receiving DAPT, a sample size of 8200 was chosen to detect a relative reduction of at least 28% with ticagrelor monotherapy and a type I error of 0. 05. Assuming a one-year rate of 8. 0% for the key secondary endpoint, a sample size of 8200 provided 80% power to exclude an absolute non-inferiority margin of 1. 6% with type I error 0. 025. An enrolled cohort of 9000 was chosen to accommodate an approximate 10% rate of randomization ineligibility.

Statistical Analysis The cumulative incidences of the primary and key secondary endpoints were expressed as Kaplan. Meier estimates by treatment group. Hazard ratios (HR) and 95% confidence intervals (CI) were generated using Cox proportional hazards models with treatment allocation as a single covariate. Treatment effects were also expressed as absolute risk differences using the one-year Kaplan-Meier estimates and Greenwood standard errors. Superiority testing using a log-rank p-value was performed for the primary endpoint. For the key secondary endpoint a one-sided non-inferiority test was performed using the upper limit of the 95% CI for the absolute risk difference. The primary endpoint was evaluated in the intention to treat (ITT) cohort while the key secondary endpoint was assessed in the per protocol cohort.

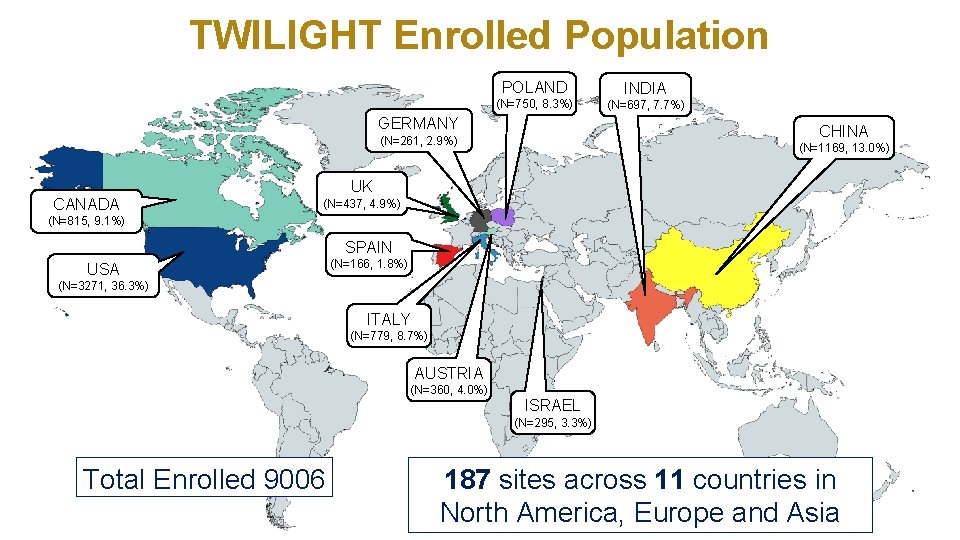
TWILIGHT Enrolled Population POLAND INDIA (N=750, 8. 3%) (N=697, 7. 7%) GERMANY CHINA (N=261, 2. 9%) CANADA (N=1169, 13. 0%) UK (N=437, 4. 9%) (N=815, 9. 1%) SPAIN USA (N=166, 1. 8%) (N=3271, 36. 3%) ITALY (N=779, 8. 7%) AUSTRIA (N=360, 4. 0%) ISRAEL (N=295, 3. 3%) Total Enrolled 9006 187 sites across 11 countries in North America, Europe and Asia

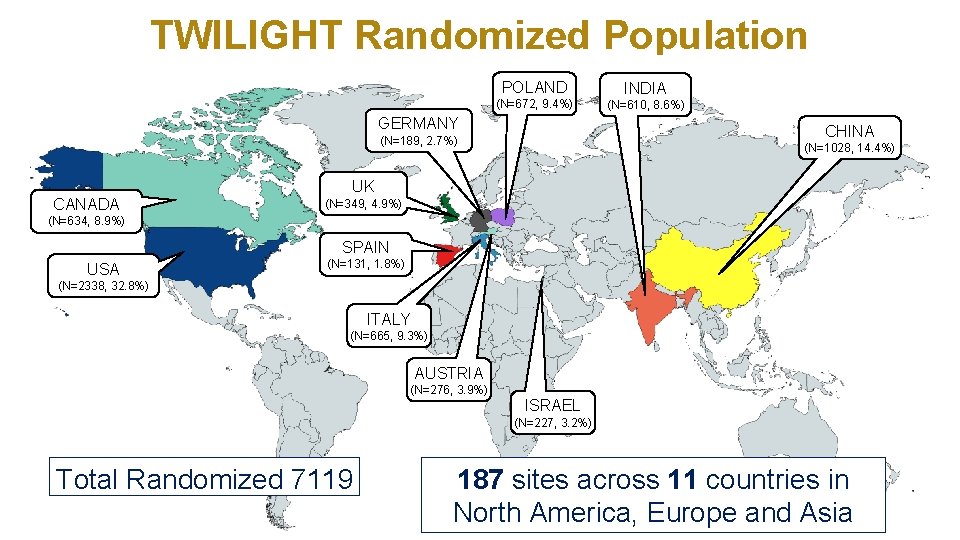
TWILIGHT Randomized Population POLAND INDIA (N=672, 9. 4%) (N=610, 8. 6%) GERMANY CHINA (N=189, 2. 7%) CANADA (N=1028, 14. 4%) UK (N=349, 4. 9%) (N=634, 8. 9%) SPAIN USA (N=131, 1. 8%) (N=2338, 32. 8%) ITALY (N=665, 9. 3%) AUSTRIA (N=276, 3. 9%) ISRAEL (N=227, 3. 2%) Total Randomized 7119 187 sites across 11 countries in North America, Europe and Asia

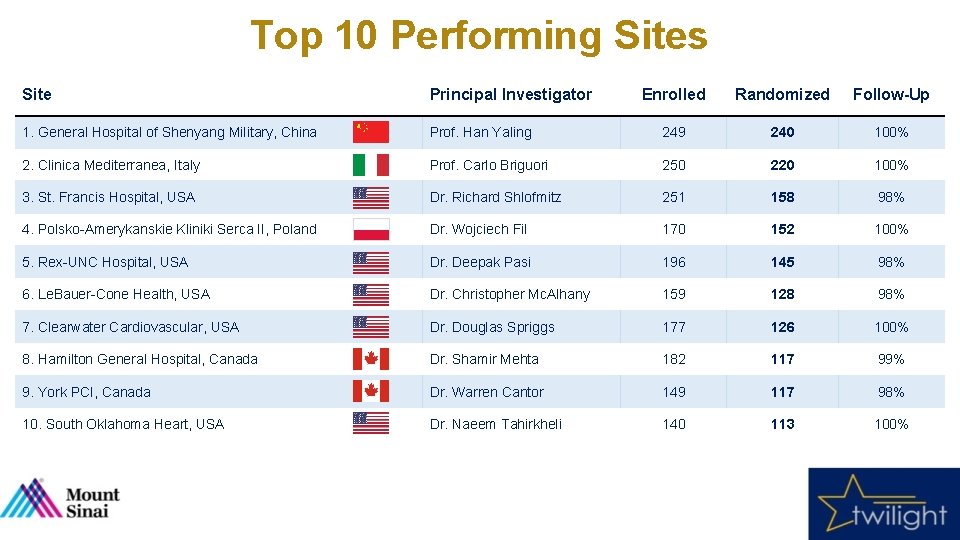
Top 10 Performing Sites Site Principal Investigator Enrolled Randomized Follow-Up 1. General Hospital of Shenyang Military, China Prof. Han Yaling 249 240 100% 2. Clinica Mediterranea, Italy Prof. Carlo Briguori 250 220 100% 3. St. Francis Hospital, USA Dr. Richard Shlofmitz 251 158 98% 4. Polsko-Amerykanskie Kliniki Serca II, Poland Dr. Wojciech Fil 170 152 100% 5. Rex-UNC Hospital, USA Dr. Deepak Pasi 196 145 98% 6. Le. Bauer-Cone Health, USA Dr. Christopher Mc. Alhany 159 128 98% 7. Clearwater Cardiovascular, USA Dr. Douglas Spriggs 177 126 100% 8. Hamilton General Hospital, Canada Dr. Shamir Mehta 182 117 99% 9. York PCI, Canada Dr. Warren Cantor 149 117 98% 10. South Oklahoma Heart, USA Dr. Naeem Tahirkheli 140 113 100%

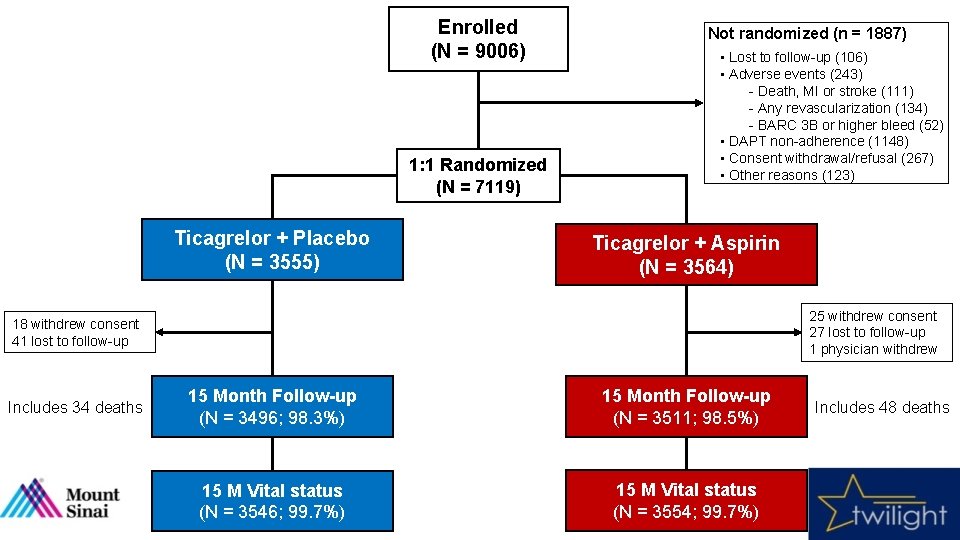
Enrolled (N = 9006) 1: 1 Randomized (N = 7119) Ticagrelor + Placebo (N = 3555) Not randomized (n = 1887) • Lost to follow-up (106) • Adverse events (243) - Death, MI or stroke (111) - Any revascularization (134) - BARC 3 B or higher bleed (52) • DAPT non-adherence (1148) • Consent withdrawal/refusal (267) • Other reasons (123) Ticagrelor + Aspirin (N = 3564) 25 withdrew consent 27 lost to follow-up 1 physician withdrew 18 withdrew consent 41 lost to follow-up Includes 34 deaths 15 Month Follow-up (N = 3496; 98. 3%) 15 Month Follow-up (N = 3511; 98. 5%) 15 M Vital status (N = 3546; 99. 7%) 15 M Vital status (N = 3554; 99. 7%) Includes 48 deaths

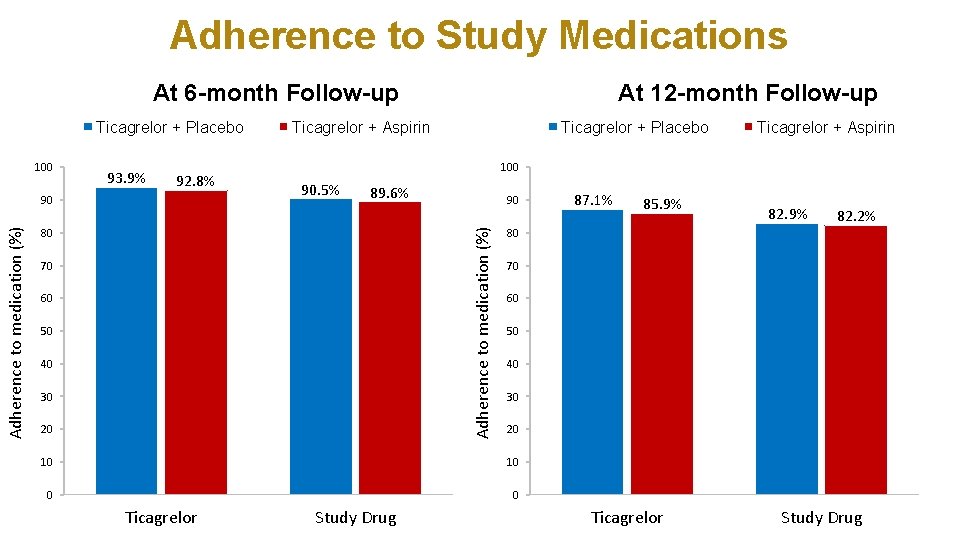
Adherence to Study Medications At 6 -month Follow-up Ticagrelor + Placebo 100 93. 9% 92. 8% Ticagrelor + Aspirin Ticagrelor + Placebo 90. 5% 89. 6% 80 70 60 50 40 30 20 90 85. 9% 82. 2% 70 60 50 40 30 20 10 0 0 Study Drug 87. 1% 80 10 Ticagrelor + Aspirin 100 Adherence to medication (%) 90 At 12 -month Follow-up Ticagrelor Study Drug

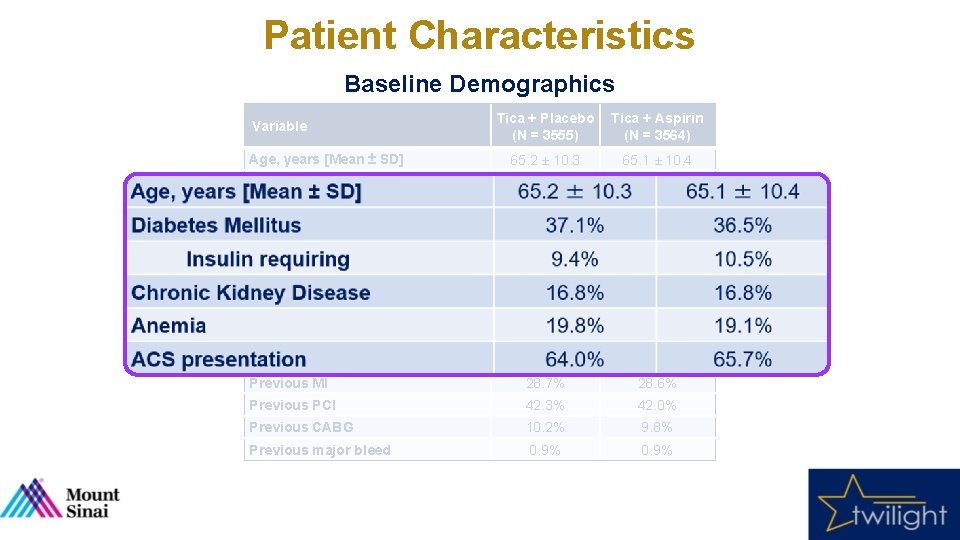
Patient Characteristics Baseline Demographics Tica + Placebo (N = 3555) Tica + Aspirin (N = 3564) 65. 2 ± 10. 3 65. 1 ± 10. 4 Female sex 23. 8% 23. 9% Nonwhite race 31. 2% 30. 5% 28. 6 ± 5. 5 28. 5 ± 5. 6 37. 1% 36. 5% 9. 4% 10. 5% Chronic Kidney Disease 16. 8% Anemia 19. 8% 19. 1% ACS presentation 64. 0% 65. 7% Current Smoker 20. 4% 23. 1% Previous MI 28. 7% 28. 6% Previous PCI 42. 3% 42. 0% Previous CABG 10. 2% 9. 8% Previous major bleed 0. 9% Variable Age, years [Mean ± SD] BMI, kg/m 2 Diabetes Mellitus Insulin requiring

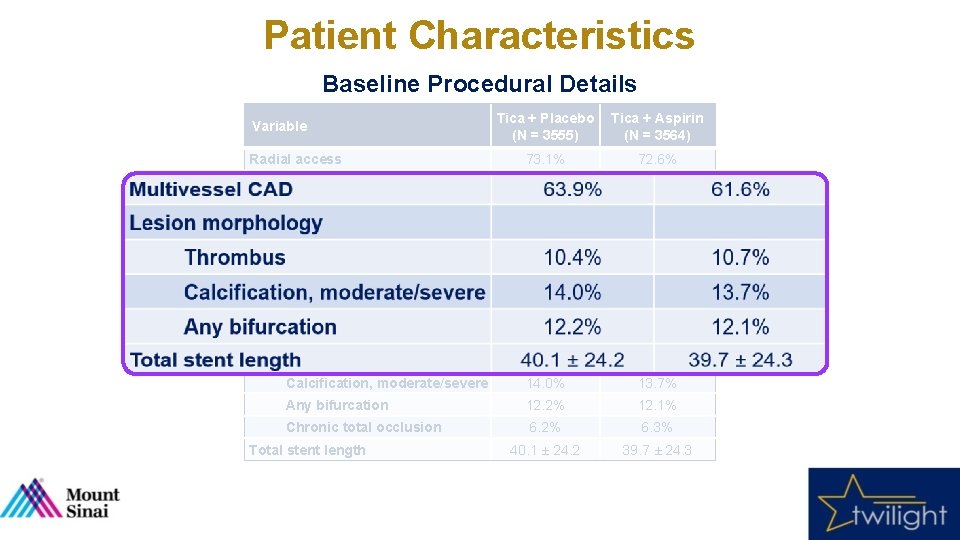
Patient Characteristics Baseline Procedural Details Tica + Placebo (N = 3555) Tica + Aspirin (N = 3564) Radial access 73. 1% 72. 6% Multivessel CAD 63. 9% 61. 6% LAD 75. 1% 74. 3% RCA 53. 5% 52. 5% LCX 46. 8% 46. 2% 7. 9% 8. 6% 1. 5 ± 0. 7 Thrombus 10. 4% 10. 7% Calcification, moderate/severe 14. 0% 13. 7% Any bifurcation 12. 2% 12. 1% Chronic total occlusion 6. 2% 6. 3% 40. 1 ± 24. 2 39. 7 ± 24. 3 Variable Target vessel Left Main Disease ≥ 50% Number of lesions treated Lesion morphology Total stent length

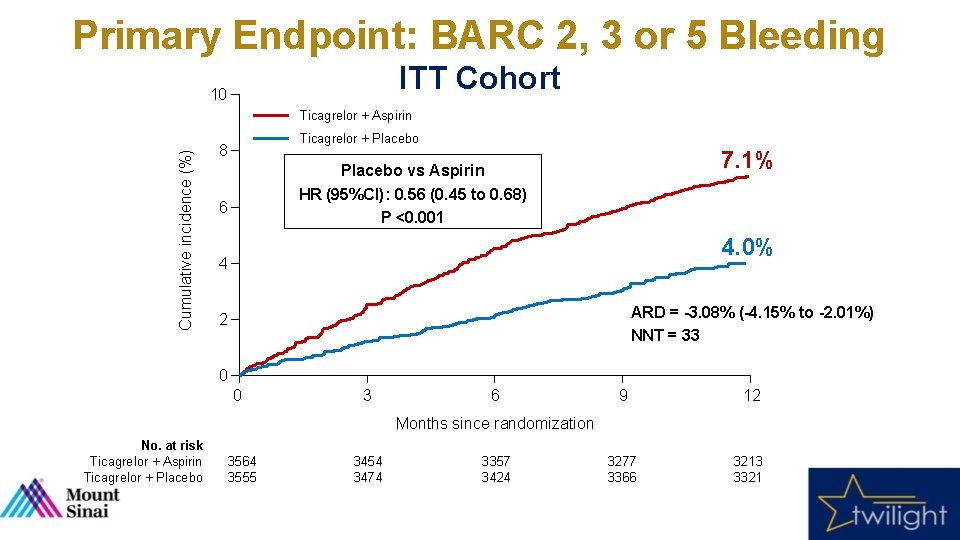
Primary Endpoint: BARC 2, 3 or 5 Bleeding ITT Cohort 10 Cumulative incidence (%) Ticagrelor + Aspirin Ticagrelor + Placebo 8 7. 1% Placebo vs Aspirin HR (95%CI): 0. 56 (0. 45 to 0. 68) P <0. 001 6 4. 0% 4 ARD = -3. 08% (-4. 15% to -2. 01%) NNT = 33 2 0 0 3 6 9 12 3277 3366 3213 3321 Months since randomization No. at risk Ticagrelor + Aspirin Ticagrelor + Placebo 3564 3555 3454 3474 3357 3424

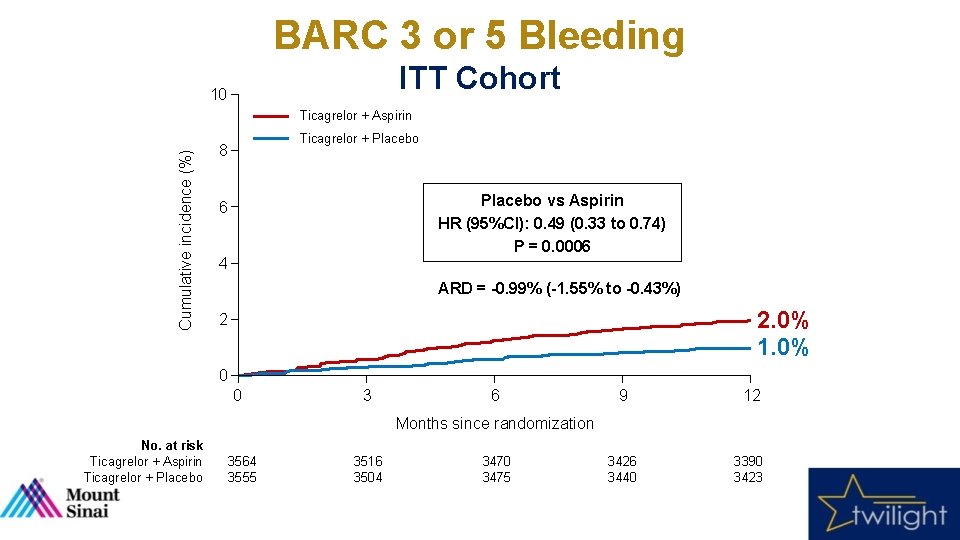
BARC 3 or 5 Bleeding ITT Cohort 10 Cumulative incidence (%) Ticagrelor + Aspirin Ticagrelor + Placebo 8 Placebo vs Aspirin HR (95%CI): 0. 49 (0. 33 to 0. 74) P = 0. 0006 6 4 ARD = -0. 99% (-1. 55% to -0. 43%) 2. 0% 1. 0% 2 0 0 3 6 9 12 3426 3440 3390 3423 Months since randomization No. at risk Ticagrelor + Aspirin Ticagrelor + Placebo 3564 3555 3516 3504 3470 3475

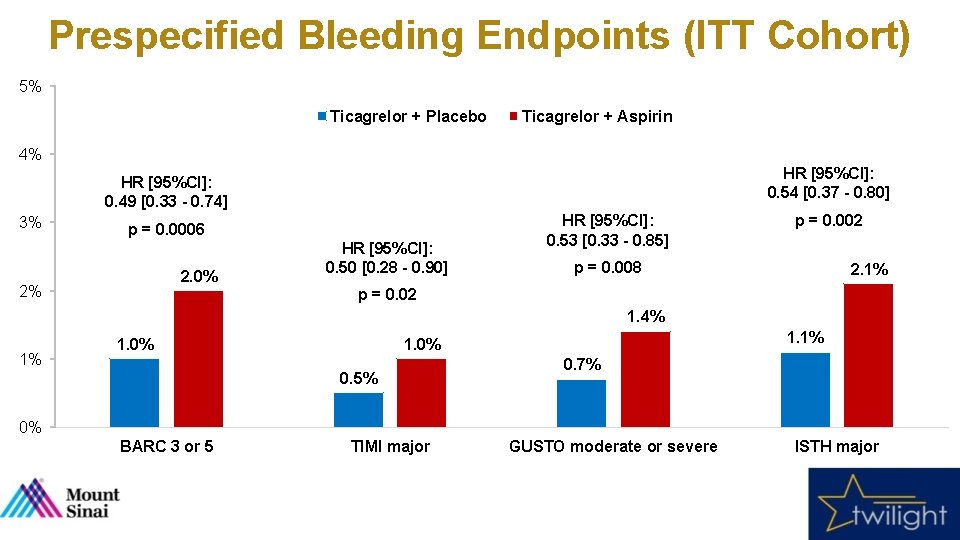
Prespecified Bleeding Endpoints (ITT Cohort) 5% Ticagrelor + Placebo Ticagrelor + Aspirin 4% HR [95%CI]: 0. 54 [0. 37 - 0. 80] HR [95%CI]: 0. 49 [0. 33 - 0. 74] 3% p = 0. 0006 2. 0% 2% HR [95%CI]: 0. 50 [0. 28 - 0. 90] HR [95%CI]: 0. 53 [0. 33 - 0. 85] p = 0. 002 p = 0. 008 2. 1% p = 0. 02 1. 4% 1% 1. 0% 1. 1% 1. 0% 0. 5% 0. 7% 0% BARC 3 or 5 TIMI major GUSTO moderate or severe ISTH major

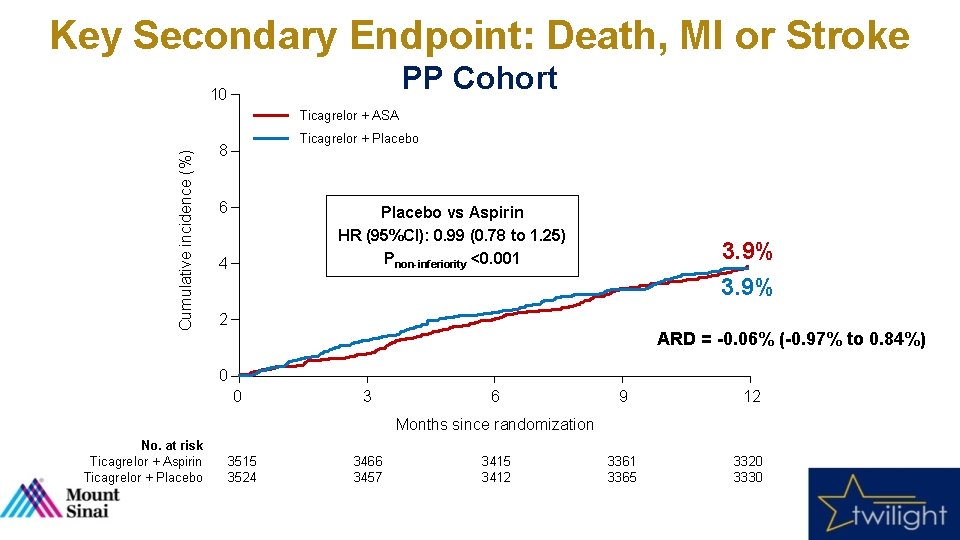
Key Secondary Endpoint: Death, MI or Stroke PP Cohort 10 Cumulative incidence (%) Ticagrelor + ASA Ticagrelor + Placebo 8 6 Placebo vs Aspirin HR (95%CI): 0. 99 (0. 78 to 1. 25) Pnon-inferiority <0. 001 4 3. 9% 2 ARD = -0. 06% (-0. 97% to 0. 84%) 0 0 3 6 9 12 3361 3365 3320 3330 Months since randomization No. at risk Ticagrelor + Aspirin Ticagrelor + Placebo 3515 3524 3466 3457 3415 3412
![Prespecified Ischemic Endpoints PP Cohort 5 HR 95CI 0 97 0 76 1 Prespecified Ischemic Endpoints (PP Cohort) 5% HR [95%CI]: 0. 97 [0. 76 - 1.](https://slidetodoc.com/presentation_image_h2/6498e48bdbb3b783eae0b646eb1df027/image-26.jpg)
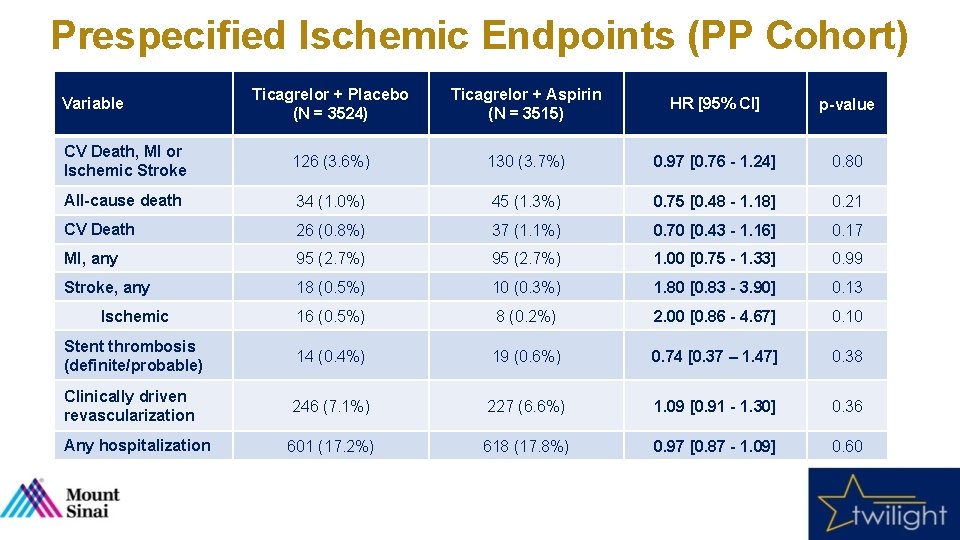
Prespecified Ischemic Endpoints (PP Cohort) 5% HR [95%CI]: 0. 97 [0. 76 - 1. 24] Ticagrelor + Placebo p = 0. 80 4% HR [95%CI]: 1. 00 [0. 75 - 1. 33] 3. 6% 3. 7% 3% Ticagrelor + Aspirin p = 0. 99 HR [95%CI]: 0. 75 [0. 48 - 1. 18] 2. 7% HR [95%CI]: 1. 80 [0. 83 - 3. 90] p = 0. 21 2% 1. 0% 1% 1. 3% p = 0. 13 0. 5% 0. 3% HR [95%CI]: 0. 74 [0. 37 – 1. 47] p = 0. 38 0. 4% 0. 6% 0% CV Death, MI or Ischemic Stroke All-cause Death MI, any Stroke, any Stent thrombosis (definite/probable)

Prespecified Ischemic Endpoints (PP Cohort) Ticagrelor + Placebo (N = 3524) Ticagrelor + Aspirin (N = 3515) HR [95% CI] p-value CV Death, MI or Ischemic Stroke 126 (3. 6%) 130 (3. 7%) 0. 97 [0. 76 - 1. 24] 0. 80 All-cause death 34 (1. 0%) 45 (1. 3%) 0. 75 [0. 48 - 1. 18] 0. 21 CV Death 26 (0. 8%) 37 (1. 1%) 0. 70 [0. 43 - 1. 16] 0. 17 MI, any 95 (2. 7%) 1. 00 [0. 75 - 1. 33] 0. 99 Stroke, any 18 (0. 5%) 10 (0. 3%) 1. 80 [0. 83 - 3. 90] 0. 13 Ischemic 16 (0. 5%) 8 (0. 2%) 2. 00 [0. 86 - 4. 67] 0. 10 Stent thrombosis (definite/probable) 14 (0. 4%) 19 (0. 6%) 0. 74 [0. 37 – 1. 47] 0. 38 Clinically driven revascularization 246 (7. 1%) 227 (6. 6%) 1. 09 [0. 91 - 1. 30] 0. 36 Any hospitalization 601 (17. 2%) 618 (17. 8%) 0. 97 [0. 87 - 1. 09] 0. 60 Variable

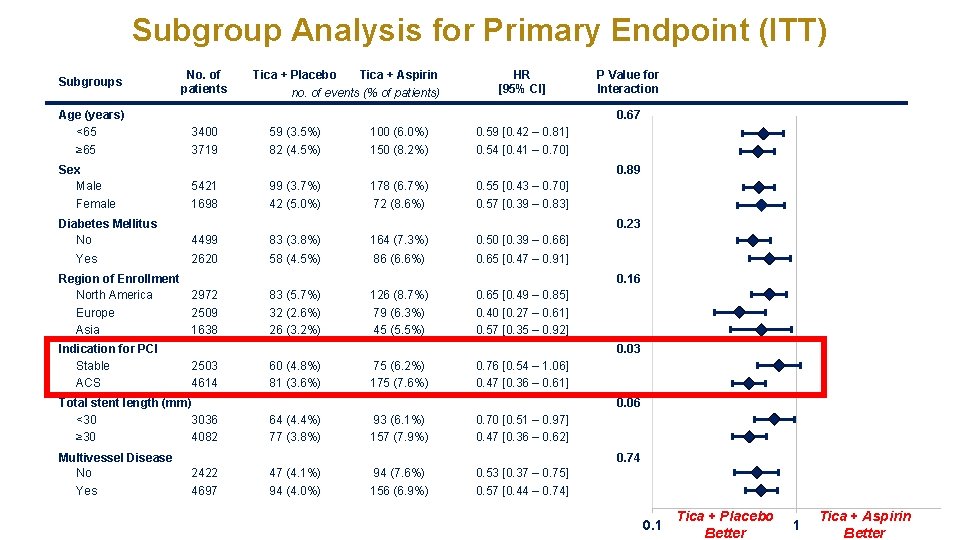
Subgroup Analysis for Primary Endpoint (ITT) Subgroups Age (years) <65 ≥ 65 Sex Male Female Diabetes Mellitus No Yes No. of patients no. of events (% of patients) HR [95% CI] 3400 59 (3. 5%) 100 (6. 0%) 0. 59 [0. 42 – 0. 81] 3719 82 (4. 5%) 150 (8. 2%) 0. 54 [0. 41 – 0. 70] P Value for Interaction 0. 89 5421 99 (3. 7%) 178 (6. 7%) 0. 55 [0. 43 – 0. 70] 1698 42 (5. 0%) 72 (8. 6%) 0. 57 [0. 39 – 0. 83] 4499 83 (3. 8%) 164 (7. 3%) 0. 50 [0. 39 – 0. 66] 2620 58 (4. 5%) 86 (6. 6%) 0. 65 [0. 47 – 0. 91] 0. 23 0. 16 83 (5. 7%) 32 (2. 6%) 26 (3. 2%) 126 (8. 7%) 79 (6. 3%) 45 (5. 5%) 0. 65 [0. 49 – 0. 85] 0. 40 [0. 27 – 0. 61] 0. 57 [0. 35 – 0. 92] 0. 03 2503 4614 Total stent length (mm) <30 3036 ≥ 30 4082 Multivessel Disease No Yes Tica + Aspirin 0. 67 Region of Enrollment North America 2972 Europe 2509 Asia 1638 Indication for PCI Stable ACS Tica + Placebo 60 (4. 8%) 81 (3. 6%) 75 (6. 2%) 175 (7. 6%) 0. 76 [0. 54 – 1. 06] 0. 47 [0. 36 – 0. 61] 0. 06 64 (4. 4%) 77 (3. 8%) 93 (6. 1%) 157 (7. 9%) 0. 70 [0. 51 – 0. 97] 0. 47 [0. 36 – 0. 62] 0. 74 2422 4697 47 (4. 1%) 94 (4. 0%) 94 (7. 6%) 156 (6. 9%) 0. 53 [0. 37 – 0. 75] 0. 57 [0. 44 – 0. 74] 0. 1 Tica + Placebo Better 1 Tica + Aspirin Better

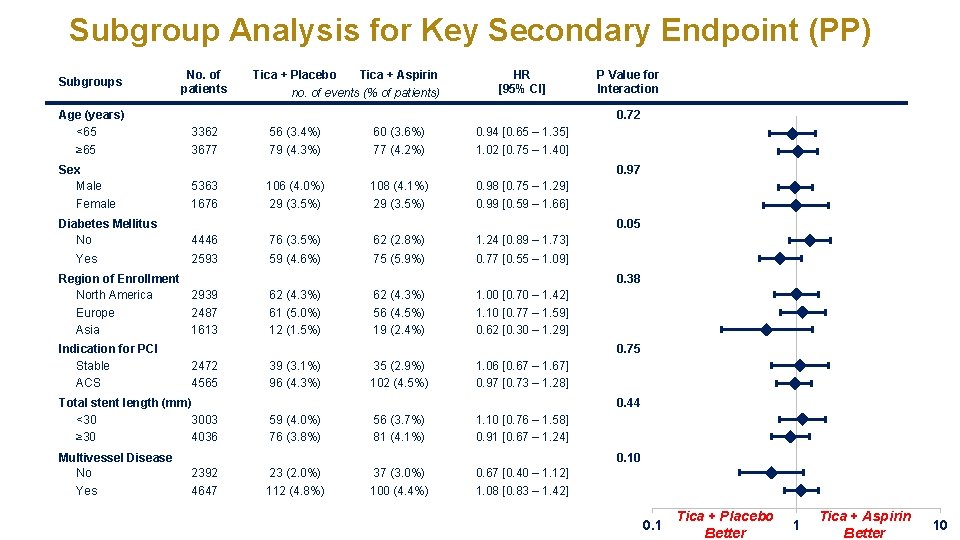
Subgroup Analysis for Key Secondary Endpoint (PP) Subgroups Age (years) <65 ≥ 65 Sex Male Female Diabetes Mellitus No Yes No. of patients no. of events (% of patients) HR [95% CI] 3362 56 (3. 4%) 60 (3. 6%) 0. 94 [0. 65 – 1. 35] 3677 79 (4. 3%) 77 (4. 2%) 1. 02 [0. 75 – 1. 40] P Value for Interaction 0. 97 5363 106 (4. 0%) 108 (4. 1%) 0. 98 [0. 75 – 1. 29] 1676 29 (3. 5%) 0. 99 [0. 59 – 1. 66] 4446 76 (3. 5%) 62 (2. 8%) 1. 24 [0. 89 – 1. 73] 2593 59 (4. 6%) 75 (5. 9%) 0. 77 [0. 55 – 1. 09] 0. 05 0. 38 62 (4. 3%) 61 (5. 0%) 12 (1. 5%) 62 (4. 3%) 56 (4. 5%) 19 (2. 4%) 1. 00 [0. 70 – 1. 42] 1. 10 [0. 77 – 1. 59] 0. 62 [0. 30 – 1. 29] 0. 75 2472 4565 Total stent length (mm) <30 3003 ≥ 30 4036 Multivessel Disease No Yes Tica + Aspirin 0. 72 Region of Enrollment North America 2939 Europe 2487 Asia 1613 Indication for PCI Stable ACS Tica + Placebo 39 (3. 1%) 96 (4. 3%) 35 (2. 9%) 102 (4. 5%) 1. 06 [0. 67 – 1. 67] 0. 97 [0. 73 – 1. 28] 0. 44 59 (4. 0%) 76 (3. 8%) 56 (3. 7%) 81 (4. 1%) 1. 10 [0. 76 – 1. 58] 0. 91 [0. 67 – 1. 24] 0. 10 2392 4647 23 (2. 0%) 112 (4. 8%) 37 (3. 0%) 100 (4. 4%) 0. 67 [0. 40 – 1. 12] 1. 08 [0. 83 – 1. 42] 0. 1 Tica + Placebo Better 1 Tica + Aspirin Better 10

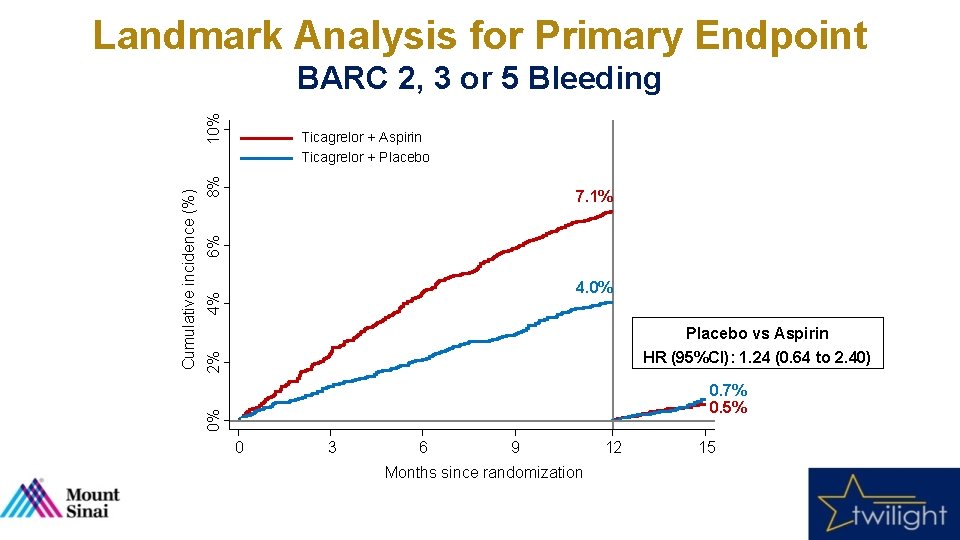
Landmark Analysis for Primary Endpoint 8% Ticagrelor + Aspirin Ticagrelor + Placebo 6% 7. 1% 4% 4. 0% 2% Placebo vs Aspirin HR (95%CI): 1. 24 (0. 64 to 2. 40) 0. 7% 0. 5% 0% Cumulative incidence (%) 10% BARC 2, 3 or 5 Bleeding 0 3 6 9 Months since randomization 12 15

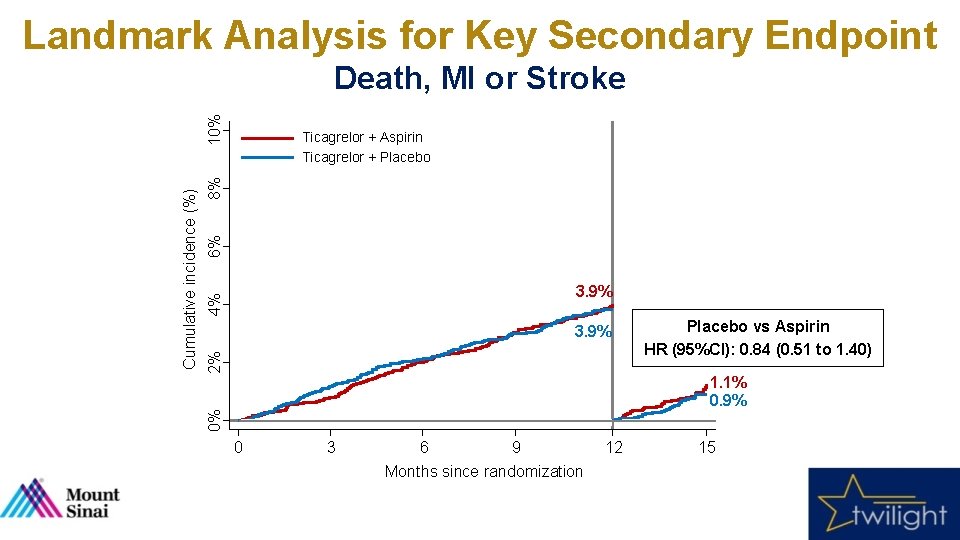
Landmark Analysis for Key Secondary Endpoint 6% 8% Ticagrelor + Aspirin Ticagrelor + Placebo 4% 3. 9% 2% 3. 9% Placebo vs Aspirin HR (95%CI): 0. 84 (0. 51 to 1. 40) 1. 1% 0. 9% 0% Cumulative incidence (%) 10% Death, MI or Stroke 0 3 6 9 Months since randomization 12 15

Limitations • These results may not be generalizable to § all patients undergoing PCI, given the requirement in our trial for both high-risk (clinical and angiographic) features, and § patients receiving background therapy with other P 2 Y 12 inhibitors. • Observed treatment effects do not apply to all enrolled participants but rather to those patients who were able to take 3 months of dual antiplatelet therapy without any major adverse events. • A lower-than expected incidence of the composite end point of death, MI, or stroke may have biased our results for this key secondary end point toward the null. • Lack of power to detect differences in the risk of important yet rare clinical events, such as stent thrombosis and stroke.

Conclusions In high-risk patients who underwent PCI and were treated with ticagrelor and aspirin for 3 months without any major adverse (bleeding or ischemic) events, an antiplatelet strategy of continuing ticagrelor monotherapy resulted in: • substantially less bleeding than ticagrelor plus aspirin • without increasing ischemic events over a period of 1 year


Acknowledgement We thank all country leaders, investigators, coordinators and study participants who made TWILIGHT possible!


COLLABORATORS Scientific Oversight: Executive Committee Roxana Mehran MD (Study Chair, Global Principal Investigator) Usman Baber MD, MS (Chair of Clinical Data Coordinating Center) Dominick J. Angiolillo MD, Ph. D David J. Cohen MD, MSc George Dangas MD, Ph. D C. Michael Gibson MD, MS Adnan Kastrati MD Mitchell Krucoff MD Global Partners Shamir Regulatory Mehta MD, MSc ACORN E. Magnus Ohman. Ph. D MD(Managing Director); Danica Cvetkovic MD, Ph. D (Medical Manager); John Gemma Robinson Mc. Entyre Ph. D (Senior Regulatory Affairs Advisor) Philippe Gabriel Steg MD Usman Baber MD, MS (Director of Clinical Biometrics); Theresa Franklin-Bond PA, MS (Director of Research Operations, Quality Outcomes & Event Adjudication); Katharine Idrissi (Director of Research Administration); Rebecca Ortega (Director of Business Development) Project Management Jerusa Altema (Clinical Research Manager); Precious Akinsanya (Clinical Research Manager); Kristin Falciglia (Project Manager); Johnston Grier (Project Manager); Pamela Kivitz (Project MARSH Manager); Carlos Bazan MD (Senior Associate); Lauren Joyce (Senior Sarah Goodman (Vice President of Life. Clinical Sciences. Research –Casualty) Clinical Research Associate); Elvira Oseledchyk MD (Senior Clinical Research Associate); Clayton Devices Snyder Consultancy (Senior Clinical Medical Ltd. Research Associate); Julia Carpenedo (Clinical Research Associate); Stefan Dabic (Clinical Research(Senior Associate); Lauren Lopez (Clinical Research Lynn Vandertie (Director); Emma Whittaker Clinical Research Associate); Kate Allen (Data Analyst) Associate); Sarah Mirza (Clinical Research Associate); Alyssa Ramkissoon (Clinical Research Associate); Shireen Wellington (Clinical Research Associate); Tiffany Moore (Publications Medmarc Astra. Zeneca and Fellowship Coordinator); Sulman Arshad (Volunteer) Scientific Oversight: Steering Committee Jeff Stroud (Life Sciences Underwriting Executive) Eva MD (Executive Director of Global Medical Affairs, Brilinta Team Leader); Chris Hewitt Paul. Turgonyi Gurbel MD MD (Global Medical Christian Hamm MDAffairs Leader Cardiovascular); Jonathan Lu MD, Ph. D (Medical Director); Gunnar Medical Oversight Pharma. Science Brandrup-Wognsen MD, Ph. D (Brilinta Medical Director Global); Narinder Bhalla MD (Head of US Davide Cao MD (Senior Post-Doctoral Research Fellow); Bimmer Claessen MD, Ph. D (Senior Timothy D. Henry MD Sharon Keays (Clinical Study Manager, Operations) Medical Affairs Cardiovascular); Barry Reicher MD, MBA (Medical Director of Clinical Research, Post-Doctoral Research Fellow); Jaya. Clinical Chandrasekhar MD, MS (Publications Manager, Senior David J. Moliterno MD CVMD); James Ferguson MD (Vice President, CVMD and Scientific External Relations); Rodrigo Gribble Post-Doctoral Research Fellow); Michela Faggioni MD (Post-Doctoral Research Fellow); Samin Sharma MD RUNDO International Pharmaceutical Research and Development Co. , Ltd. MS, MBA (Vice President of Brilinta & Epanova); Naeem Khan MD (Vice President- CVMD); Lois Serdar Farhan MD (Post-Doctoral Research Fellow); Gennaro Giustino MD (Post-Doctoral Bo Qin (Project Manager); Yoyo Wang (Project Manager); Dan Zheng (Project Manager) Walker BSN, MPH (External Scientific Research Manager); Vitalina Rozenfeld Pharm. D. (Medical Research Fellow); Sabato Sorrentino MD, Ph. D (Post-Doctoral Research Fellow); Birgit Vogel Scientific Oversight: Country Leaders Science Liaison) MD (Post-Doctoral Research Fellow); Rishi Chandiramani MD (Post-Doctoral Research Prof. Bernhard Witzenbichler MD (Germany) Salud Madrid, Hospital Universitario Clinical San Carlos Scholar); Sherif Elsayed MD (Post-Doctoral Research Scholar); Zhen Ge MD (Post-Doctoral Vladimir Dzavik MD (Canada) Bay Area Health Trust (or Bay Area Research Logistics (BARL) Prof. Javier Escaned MD (Spain Country Leader, Site Principal Investigator); Sarah Fernandez (Site Research Scholar); MDPozzi (Post-Doctoral Research Scholar); Paul Guedeney MD Prof. Javier Escaned MD (Spain) Paul Mc. Cracken CPA (Business Unit Director); Gina Howley (Production Coordinator); Marlena Coordinator); Maria Ridhima Aranzazu. Goel Ortega (Site Coordinator) (Post-Doctoral Research Scholar); Yuqi Liu MD (Post-Doctoral Research Scholar); Anastasios Prof. Robert Gil MD, Prof. Dariusz Dudek MD, Ph. D (Poland) Nagorski (Clinical Trial Coordinator); Roumeliotis MD (Post-Doctoral Research Scholar); Moritz Blum BS (Research Scholar) Prof. Kurt Huber MD (Austria) Shaare Zedek Upendra. Health Kaul MD (India) Cardinal Pharmaceutical Co. , Ltd. Dmitry Dratva MD (Site Principal Investigator); Astrid Rojansky (Site Coordinator) Biostatistics Vijay. Meng Kunadian MD, Trial Prof. Keith Oldroyd MD (United Kingdom) Ying (Clinical Associate); Yueyue Zhang (Clinical Trial Associate) Samantha Sartori Ph. D (Biostatistician); Melissa Aquino MS (Data Science Analyst III); Rachel Prof. Gennaro Sardella MD (Italy) SHARP Clinical Devices Singleton MS (Data Science Analyst I); Sonny Sayseng MS (Database Michele Giora. Service Weisz Corporation MD, Ran Kornowski MD (Israel) FOJP Peter Tomlinson (Logistics Project Manager); Hannah Williams (Logistics. Administrator); Project Manager) Sarin MS (Programmer Analyst I) Prof. Han Ya-Ling(Vice MD (China) Charles Ferguson President of Client Services); Duane Perricelli (Vice President of Risk Management Advisory Services); Andrea Rienzo (Mount Sinai Representative) The Newcastle Upon Tyne Hospitals, NHS Hospital Trust Vijay Kunadian MD (United Kingdom Country Leader, Site Principal Investigator); Natasha Bridgeman Pharmacovigilance & Safety Data Monitoring Committee Helios Amper-Klinikum, Dachau (REC Manager); Lesley Hall Ph. D Theresa Franklin-Bond PA, MS(Senior Trial Manager); Phil Mawson (R & D Study Manager) Bernard J. Gersh MB, Ch. B, DPhil (Chair, Data Safety Monitoring Board) Prof. Bernhard Witzenbichler MD (Germany Country Leader, Site Principal Investigator); Marion Timothy Collier MSc (Independent Statistician) Senegewald (Site. MD Coordinator) Swoboda (Site Coordinator) Trialog Ltd. Management Clinical Events. Trials, Committee David P. Faxon (Member, Marlene Data Safety Monitoring Board) Tamar Itzhaky (Senior Returns Destruction Specialist); Ossad(Clinical (Senior Events Import Associate); Coordinator); Jin Young Cha (Clinical Eventsand Project Manager II); Rashi. Jean Bedekar Spencer B. King MD (Member, Data Safety Monitoring Board) i. Process Global Research Inc. Revital Aksakalov (Pharmacist); Tally Levin (Import Coordinator) Raza Hijazi (Clinical Events Associate); Mike Koh (Clinical Events Associate); Kamilia Moalem Stuart Pocock Ph. D (Member, Data Safety Monitoring Board) Asker (Director); Mohammed Operations James. Ahmed E. Tcheng MD (Member, Data. Saleem Safety (Clinical Monitoring Board) Manager); Deepa Ganesh (Senior (Clinical Events Associate); Amika Purushotham (Clinical Events Associate); Zaha Waseem Clinical Research Associate); Abhinaya Bhatta MD (Clinical Research Associate); Karthik Ramasamy Well. Spring Pharma Services (Clinical Events Associate); Emma Woodoff-Leith (Clinical Events Associate); Jayden Clinical Events Committee

SITE LIST and INVESTIGATORS Principal Investigator Dr. Piotr Palinski Prof. Kurt Huber Dr. Krzysztof Milewski Dr. Clemens Steinwender Prof. Robert Gil Dr. Aleksander Christian Ebner Dr. Żurakowski Dr. Maciej Hubert. Maliszewski Wallner Dr. Witold Anna Rab Dr. Żmuda Prof. Dariusz Stefan Harb Prof. Dudek Dr. Jacek David. Godlewski Zweiker Dr. Matthias Frick Dr. Małgorzata Głowaty Dr. Adam Franz Weidinger Dr. Kern Dr. Janusz Payam. Szczupak Dehghani Dr. Marek Shamir. Kondys Mehta Dr. Wojciech Warren Cantor Dr. Fil Dr. Jakub Mina Madan Dr. Foryś Dr. Janusz Anthony Della Siega Dr. Prokopczuk Dr. Nader Shahar. Elmasri Lavi Dr. Asim Cheema Prof. Javier Escaned Dr. Josepa Vladimir Dzavik Dr. Mauri & Dr. Omar Abdul Dr. Rafael Albert Romaguera Chan Dr. Manuel Han Ya-Ling Dr. Sabate Tenas Dr. Jose Lin Hailong Dr. Maria de la Torre Hernandez Dr. Ruiyan Zhang Prof. Keith Oldroyd Dr. John Qiu Chunguang Dr. Irving Dr. Nicholas Jianan Wang Cruden Dr. Vijay Xianghua Fu Dr. Kunadian Dr. Simon Yongjun Li Dr. Hetherington Dr. David Jiyan Chen Dr. Hildick-Smith Dr. Peter Cuilian. O’Kane Dai Dr. Ross Shaoliang Chen Dr. Mc. Geoch Dr. Scot Xi Su. Garg Dr. Peter Qiang. Ludman Wu Dr. Jonathan Ying Huang & Dr. Yitong Ma Dr. Watt Dr. Robert Shuyang Zhang Dr. Butler Dr. Andrew Jianhong. Wragg Tao Dr. Simon Suxin Luo Dr. Walsh Dr. Magdi Yujie Zhou Dr. El-Omar Dr. Marcus Xianxian. Flather Zhao Dr. Samin Yawei Sharma Xu Dr. Anthony Xiang Cheng Dr. J. Spaedy Dr. William Yingxian. Dixon Sun Dr. Mitchell Ming Cui. Rashid Dr. Thom Jianhua Zhu Dr. Dahle Dr. Michael Zaixin Yu. Foster Dr. Rohit Xiaoyong Dr. Amin. Qi Dr. Magdi Shouli Ghali Wang Dr. Dr. Michael Lianqun Kim Cui Country Poland Austria Poland Austria Poland Austria Poland Canada Poland Canada Spain Canada Spain China United Kingdom United China Kingdom China United Kingdom China United Kingdom China United Kingdom China United States China United States China United States United China States Hospital Centrum Kardiologii Allenort Scanmed - Tomaszów Mazowiecki Wilhelminen Hospital Tychy X Oddzial Kardiologii Kepler University Hospital Linz Centrum Szpital Kliniczny MSW Krankenhaus Elisabethinen Linz Gmb. H Malopolskie der Centrum Sercowo-Naczyniowe. KH Schwarzach Centrum Kardiologii Inwazyjnej- Ostrowiec Landeskrankenhaus Centrum Kardiologii. Villach Inwazyjnej-Oświęcim LKH Graz. Uniwersytecki Sud-West Krakow Szpital LKH-Univ. Klinikum Graz Centrum Kardiologii Inwazynej Pinczow Landeskrankenhaus Centrum Kardiologii. Feldkirch Allenort Scanmed, Kutno Krankenanstalt Rudolfstiftung Wojewodzki Szpital Specjalistyczny, Olsztyn Prairie Vascular Research Inc. Centrum Kardiologii Inwazyjnej, Krosno, Hamilton Sciences Mc. Master III Oddzial. Health Kardiologii Inwazyjnej, Dabrowa Gornicza Southlake Regional Health Centre Polsko-Amerykańskie Kliniki Serca II, Bielsko-Biala Sunnybrook Research Institute Zgierskie Centrum Kardiologii, Victoria Heart Institute. Inwazyjnej Foundation IV Oddzial Kardiologii Lawson Health Research Institute Gdańskie Centrum Sercowo-Naczyniowe Dixie Medical Group Hospital Clinico San Carlos University of Toronto Hospital German Trias i Pujol Royal Columbian Hospital, Fraser Clinical Trials Hospital Universitari de Bellvitge The General Hospital of Shenyang Military Hospital Clinic Barcelona Dalian Municipal Center. Marques Hospital. De Valdecilla Hospital Universitario Rui Jin Hospital Jiaotong University School of Medicine Golden Jubilee Shanghai National Hospital The First Affiliated of Zhengzhou University Ninewells Hospital (NHS Tayside) The Royal Second Infirmary Affiliated of Edinburgh Hospital(NHS Zhejiang Lothian) University The Second Hospital of Hebei University (5 th Ward) Freeman Hospital The 2 nd Hospital Hebei Medical University (4 th Ward) Kettering Generalof. Hospital Guangdong General Brighton and Sussex. Hospital University Hospitals Xiamen Cardiovascular Hospital Xiamen University Royal Bournemouth Hospital Nanjing First. Hospital(NHS Jiangsu Hairmyres Lanarkshire) Wu. Han Asia Heart. Hospital Royal Blackburn Guizhou Province Hospital People’s Hospital Queen Elizabeth The 1 st Affiliated Hospital of Xinjiang Medical University Raigmore Hospital (NHS Highland) Peking Union. University Medical College Hospital Royal Stoke Hospital People’s Hospital of. Hospital Sichuan Province St. Bartholomew's The 1 st Affiliated of Chongqing Medical University Belfast Health & Hospital Social Care Trust Beijing Hospital Central. Anzhen Manchester University Hospital Shanghai Norfolk &Changhai Norwich. Hospital University Hospital Shanghai Tenth Peoples Hospital Mount Sinai Hospital Wuhan Union Hospital Missouri Heart Center The First Hospital of China Medical University Tallahassee Research Institute Peking Third Hospital CAMC University Clinical Trials Center The First Affiliated Hospital Zhejiang University Centra. Care Heart and Vascular Center Xiangya Hospital. Heart Central South University Cardiology, LLC) South Carolina Center (Providence Hebei General Hospital Penisacola Research Consultants, Inc. The 306 th Iowa Heart. Hospital Center of PLA Shandong Lenox Hill. Provincial Hospital Dr. Barry Investigator Principal Bertolet United Country States Prof. Dr. Richard Bernhard Shlofmitz Witzenbichler United Germany States Dr. Paul Carsten Seigel Schwencke United Germany States Dr. Jonathan Alexander. Roberts Bufe United Germany States Dr. Zeshan Tobias Geisler A. Rana United Germany States Dr. Naeem Stephan. Tahirkheli Achenbach United Germany States Dr. Christopher Carsten Skurk Metzger United Germany States Dr. Habib Volker. Samady Schaechinger United Germany States Dr. Michael Christoph. Rosenberg Liebetrau United Germany States Dr. Sorin Ingo Voigt Brener & Dr. Christoph Jensen United Germany States Dr. Denes Daniel Korpas Duerschmied & Dr. Ingo Ahrens United Germany States Dr. Virender Adnan Kastrati Sethi United Germany States Dr. Jason Johannes B. Lindsey Brachmann United Germany States Dr. Aron Milind. Schwarcz Gadkari United. India States Dr. Chao-Wei Patel Tejas. Hwang Madhusudan United. India States Dr. Mansoor Sanjeev KA. Sharma Qureshi United. India States Dr. Enrique Tapan Ghose Rivera& Dr. Ripen Gupta United. India States Dr. Maria Atul Mathur Bartlett & Prof. Upendra Kaul United. India States Dr. Larry Satya Van-Thomas Gupta Crisco United. India States Dr. Samuel Prabhavathi Durr United. India States Dr. Alpesh Keshava. Shah R United. India States Dr. Ziad Neeraj Abbud Pandit United. India States Dr. John Dmitry Fox Dratva & Dr. Giora Weisz United Israel States Prof. Dr. Dominick Abid Assali Angiolillo & Prof. Morris Mosseri United Israel States Dr. Matthew Shaul Atar. Bilodeau United Israel States Dr. Majdi Gregory Halabi Giugliano United Israel States Dr. Arash Carlos Arshi Cafri United Israel States Dr. Deepak Gabriel Greenberg Pasi & Dr. Eli Lev United Israel States Prof. Dr. David Ran Kornowski Moliterno & Prof. Abid Assali United Israel States Dr. Abbas Sa’ar Minha Shehadeh United Israel States Dr. Zafir Robert A. Zukerman Hawa & Prof. Eugenia Nikolsky United Israel States Prof. Dr. Ronald Yosef Caputo Rozenman United Israel States Prof. Dr. David Yaron Rizik Arbel United Israel States Dr. Christopher Giulio Stefanini Mc. Alhany United. Italy States Dr. Guillermo Alaide Chieffo Pineda United. Italy States Dr. Louis Ciro Indolfi Kantaros United. Italy States Dr. Gennaro Robert Iwaoka Sardella United. Italy States Dr. Syed Paolo. Gilani Canova & Dr. Giuseppe Musumeci United. Italy States Dr. Douglas Roberto Spriggs Adriano Latini & Dr. Bernardo Cortese United. Italy States Dr. Thomas Alfonso Ielasi Wang United. Italy States Dr. David Carlo Briguori Bohle United. Italy States Dr. Vamsi Ferdinando Krishna Varbella United. Italy States Dr. Herbert Enrico Cerrato Aronow United. Italy States Dr. George Marco Ferlini Kichura United. Italy States Dr. Tommy Sergio Berti C. Lee United. Italy States Dr. Charles Lara Frediani O'Shaughnessy United. Italy States Hospital Cardiology Associates of North Mississippi Helios Saint Francis Amper-Klinikum Hospital Dachau MVZ South. Hamburg Florida Research Group Helios Memorial Klinikum Healthcare Krefeld. System Universitaetsklinikum Cardiology Associates Tubingen of Fredericksburg Universitaetsklinikum South Oklahoma Heart. Erlangen Research Charite Wellmont Berlin CVA Heart Institute Klinikum Emory University Fulda Kerckhoff-Klinik Holy Family Memorial Forschungsgesellschaft mb. H Elisabeth New York. Krankenhaus Presbyterian Brooklyn Methodist Universitäts-Herzzentrum Nebraska Heart Institute Freiburg Deutsches Hackensack. Herzzentrum University Medical Muenchen Center Klinikum Saint Luke's Coburg Cardiovascular Consultants KEM Englewood Hospital Cardiovascular Research Center Associates of North Jersey Apex Frederick Heart. Memorial Institute Hospital Eternal Michigan Heart Care & Research Institute Fortis Sarah Flt Cannon Lt Rajan Blake Dhall Medical Hospital Center Fortis Sarah Escort Cannon. Heart Coliseum Institute Medical Center Care Sarah. Institute Cannon of Memorial Medical Jackson Sciences. Hospital Sri Black Jayadeva Hills Cardiovascular Institute of Cardiovascular Research Sciences and Research Fortis Houston Bangalore Methodist Research Insitute Ram Meridian Manohar Health Lohia Hospital Shaare Mount Zedek Sinai Beth Medical Israel. Center Meir University Medical of Florida Center Galilee Lutheran Medical Hospital Center of Indiana Ziv Baystate Medical Center Soroka Ohio Health Medical Research Center. Institute Hasharon Rex-UNC Hospital, Medical Center Inc Belinson University. Medical of Kentucky Center. Research Foundation Assaf Saint Michael's Harofeh Medical. Center Rambam North Kansas Health City. Corporation Hospital Edith St. Joseph’s Wolfson Hospital Medical Health Center Tel Honor. Health Aviv Medical Research Center. Institute Humanitas Le. Bauer Cardiovascular Mirasole Research Foundation San Palmetto Raffaele Health Magna Hudson. Graecia Valley Heart University Center Policlinico Novant Health Umberto I ASST-Papa University of Giovanni Texas System XXIII Ospedale Clearwater. Fatebenefratelli Cardiovascular Ospedale Washington Bolognini Adventist Hospital Clinica Novant. Mediterranea Forsyth Memorial Hospital Infermi Seton Heart Rivoli. Institute Hospital. Hays San The Luigi Miriam Gonzaga Hospital Policlinico Saint Louis. San Heart Matteo and Vascular Gabriele Bakersfield Monasterio Memorial Hospital Azienda North Ohio Usl Heart Toscana Center Nord Ovest
 Classic asp to asp.net migration
Classic asp to asp.net migration Ticagrelor
Ticagrelor Ticagrelor
Ticagrelor Sihd medical abbreviation
Sihd medical abbreviation Ticagrelor
Ticagrelor Tcagrelor
Tcagrelor Ticagrelor vs prasugrel vs clopidogrel
Ticagrelor vs prasugrel vs clopidogrel Ticagrelor
Ticagrelor Ticagrelor
Ticagrelor Ticagrelor
Ticagrelor Asp.net core tutorialspoint
Asp.net core tutorialspoint Asp arcobaleno cordenons
Asp arcobaleno cordenons Internet information services (iis) 7.0
Internet information services (iis) 7.0 What is aspnet
What is aspnet Asp co to jest
Asp co to jest Syla asp
Syla asp Signe de rovsing
Signe de rovsing Savac bus
Savac bus Brunei police organizational structure
Brunei police organizational structure Fft asp
Fft asp Asp include file
Asp include file Asp custom control
Asp custom control Do while asp
Do while asp Asp 102
Asp 102 Inurl:.asp?id=shoping
Inurl:.asp?id=shoping Need for madness
Need for madness Reflection net
Reflection net Asp net roadmap
Asp net roadmap Asp net core future
Asp net core future Chilisoft asp
Chilisoft asp Inurl:to.asp?card=
Inurl:to.asp?card= Atrésie duodénale asp
Atrésie duodénale asp Asp normal
Asp normal Inurl:bug bounty intext:token of appreciation
Inurl:bug bounty intext:token of appreciation Negative prefixes
Negative prefixes Client server architecture in asp net
Client server architecture in asp net Asp.net execution model
Asp.net execution model Outsourcing asp
Outsourcing asp Validation controls in asp.net with examples
Validation controls in asp.net with examples