Ticagrelor With Asp Irin or ALone In Hi







- Slides: 19

Ticagrelor With Asp. Irin or ALone In Hi. GH-Risk Patients After Coronary In. Tervention Study Status Update Global Principal Investigator: Roxana Mehran, MD Sponsor/ARC: Icahn School of Medicine at Mount Sinai, New York, NY Funding Agency: Astra. Zeneca

Disclosures I have no relevant financial relationships

Outline • Study Rationale • Study Design • Current Study Status • Future Directions

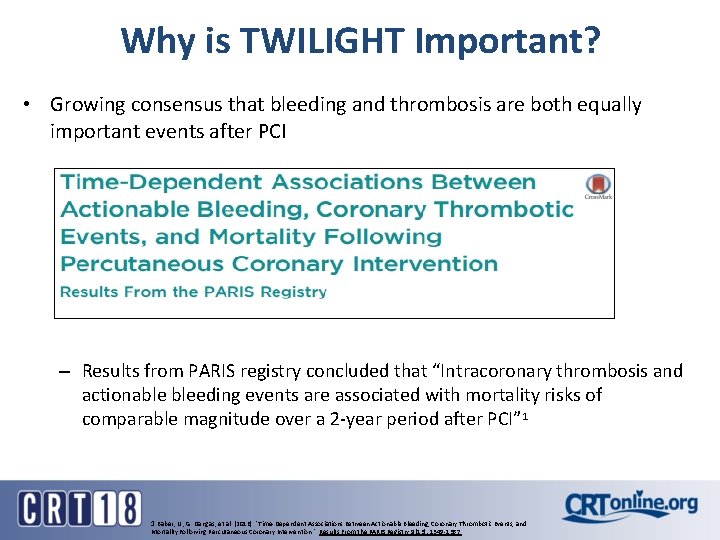
Why is TWILIGHT Important? • Growing consensus that bleeding and thrombosis are both equally important events after PCI – Results from PARIS registry concluded that “Intracoronary thrombosis and actionable bleeding events are associated with mortality risks of comparable magnitude over a 2 -year period after PCI” 1 1 Baber, U. , G. Dangas, et al. (2016). "Time-Dependent Associations Between Actionable Bleeding, Coronary Thrombotic Events, and Mortality Following Percutaneous Coronary Intervention. " Results From the PARIS Registry 9(13): 1349 -1357.

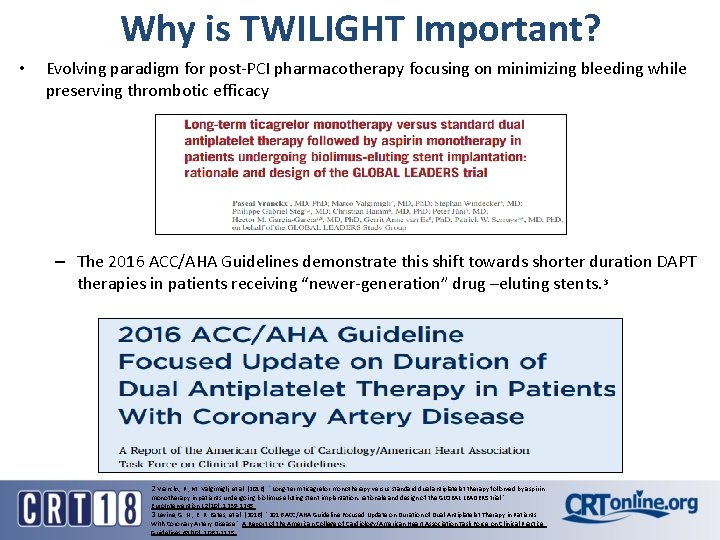
Why is TWILIGHT Important? • Evolving paradigm for post-PCI pharmacotherapy focusing on minimizing bleeding while preserving thrombotic efficacy – The 2016 ACC/AHA Guidelines demonstrate this shift towards shorter duration DAPT therapies in patients receiving “newer-generation” drug –eluting stents. 3 2 Vranckx, P. , M. Valgimigli, et al. (2016). "Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation: rationale and design of the GLOBAL LEADERS trial. " Euro. Intervention 12(10): 1239 -1245. 3 Levine, G. N. , E. R. Bates, et al. (2016). "2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease. " A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines 68(10): 1082 -1115.

Why is TWILIGHT Important? • Recently, there has been more recognition for trials that withdraw rather than add to current treatments “…we need clinical trials that can investigate the withdrawal of certain established medications to see whether such withdrawal induces patient benefit, harm, or no difference compared with continued medication. ” 4 - Stuart Pocock, Ph. D & Bernard Gersh, MB, Ch. B, DPhil 4 Pocock, S. J. and B. J. Gersh (2014). "Do Current Clinical Trials Meet Society’s Needs? " J Am Coll Cardiol 64(15): 1615 -1628.

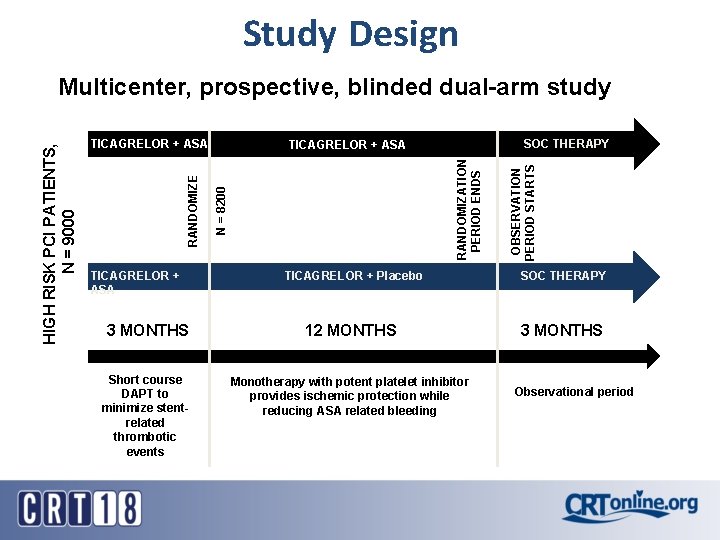
Study Design SOC THERAPY N = 8200 RANDOMIZATION PERIOD ENDS TICAGRELOR + ASA OBSERVATION PERIOD STARTS TICAGRELOR + ASA RANDOMIZE HIGH RISK PCI PATIENTS, N = 9000 Multicenter, prospective, blinded dual-arm study TICAGRELOR + Placebo SOC THERAPY 3 MONTHS 12 MONTHS 3 MONTHS Short course DAPT to minimize stentrelated thrombotic events Monotherapy with potent platelet inhibitor provides ischemic protection while reducing ASA related bleeding TICAGRELOR + ASA Observational period

INCLUSION CRITERIA Clinical Inclusion Criteria *Must meet ≥ 1 of the below Angiographic Inclusion Criteria *Must meet ≥ 1 of the below • Adult patients ≥ 65 years of age • Multi-vessel CADt • Female sex • Target lesion requiring total stent length >30 mm • Troponin positive acute coronary syndrome • Thrombotic target lesion(s) • Established Vascular Disease* • Bifurcation lesion (X, 1, 1) requiring at least 2 stents • Diabetes mellitus requiring medications • Left Main (≥ 50%) lesion OR Proximal LAD (≥ 70%) lesion CKD (e. GFR <60 ml/min/1. 73 m 2 OR Cr. Cl <60 ml/min) • Calcified target lesion(s) requiring atherectomy •

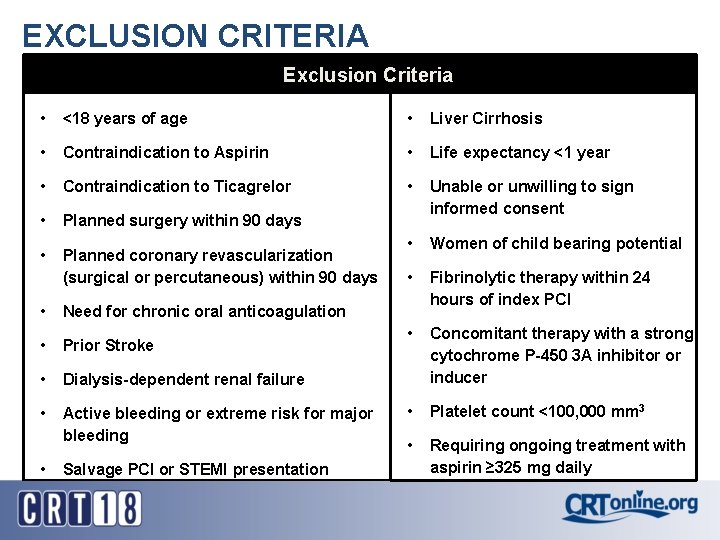
EXCLUSION CRITERIA Exclusion Criteria • <18 years of age • Liver Cirrhosis • Contraindication to Aspirin • Life expectancy <1 year • Contraindication to Ticagrelor • • Planned surgery within 90 days Unable or unwilling to sign informed consent • Planned coronary revascularization (surgical or percutaneous) within 90 days • Women of child bearing potential • Fibrinolytic therapy within 24 hours of index PCI • Concomitant therapy with a strong cytochrome P-450 3 A inhibitor or inducer • Platelet count <100, 000 mm 3 • Requiring ongoing treatment with aspirin ≥ 325 mg daily • Need for chronic oral anticoagulation • Prior Stroke • Dialysis-dependent renal failure • Active bleeding or extreme risk for major bleeding • Salvage PCI or STEMI presentation

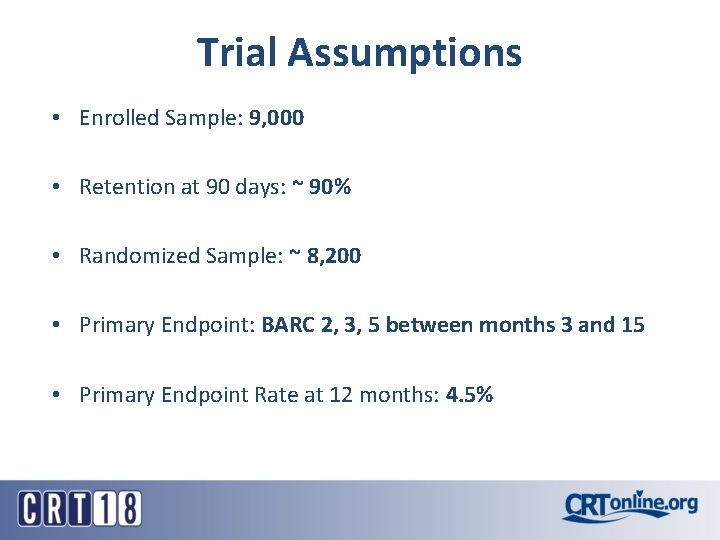
Trial Assumptions • Enrolled Sample: 9, 000 • Retention at 90 days: ~ 90% • Randomized Sample: ~ 8, 200 • Primary Endpoint: BARC 2, 3, 5 between months 3 and 15 • Primary Endpoint Rate at 12 months: 4. 5%

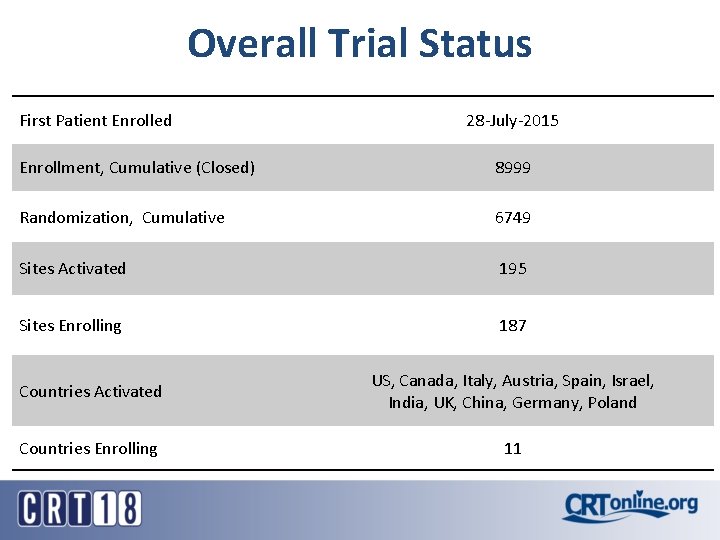
Overall Trial Status First Patient Enrolled 28 -July-2015 Enrollment, Cumulative (Closed) 8999 Randomization, Cumulative 6749 Sites Activated 195 Sites Enrolling 187 Countries Activated US, Canada, Italy, Austria, Spain, Israel, India, UK, China, Germany, Poland Countries Enrolling 11

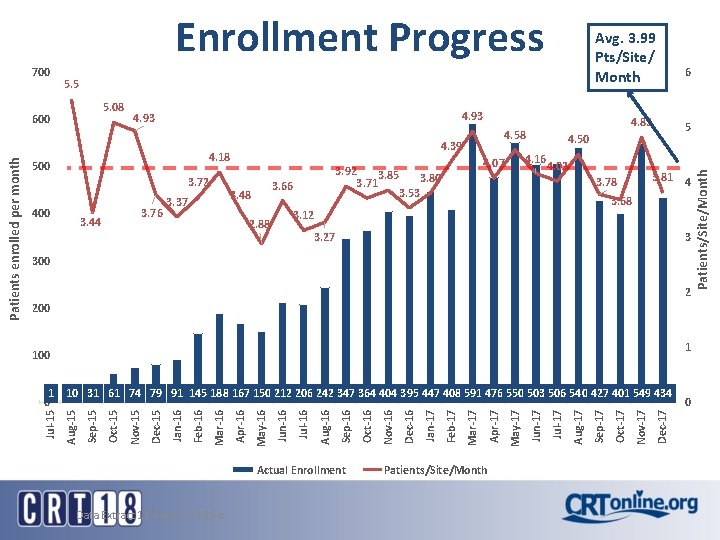
700 5. 5 5. 08 600 4. 93 3. 72 400 3. 76 3. 44 3. 48 3. 37 4. 02 3. 81 4 3. 78 3. 68 3. 12 2. 88 6 5 4. 50 4. 16 4. 07 3. 92 3. 85 3. 80 3. 71 3. 53 3. 66 4. 82 4. 58 4. 39 4. 18 500 3. 27 3 300 2 200 1 100 Actual Enrollment Data Extract 12 -Feb-17 12: 15 p Patients/Site/Month Dec-17 Nov-17 Oct-17 Sep-17 Aug-17 Jul-17 Jun-17 May-17 Apr-17 Mar-17 Feb-17 Jan-17 Dec-16 Nov-16 Oct-16 Sep-16 Aug-16 Jul-16 Jun-16 May-16 Apr-16 Mar-16 Feb-16 Jan-16 Dec-15 Nov-15 Oct-15 Sep-15 Aug-15 1 10 31 61 74 79 91 145 188 167 150 212 206 242 347 364 404 395 447 408 591 476 550 503 506 540 427 401 549 434 0 Jul-15 Patients enrolled per month Avg. 3. 99 Pts/Site/ Month 0 Patients/Site/Month Enrollment Progress

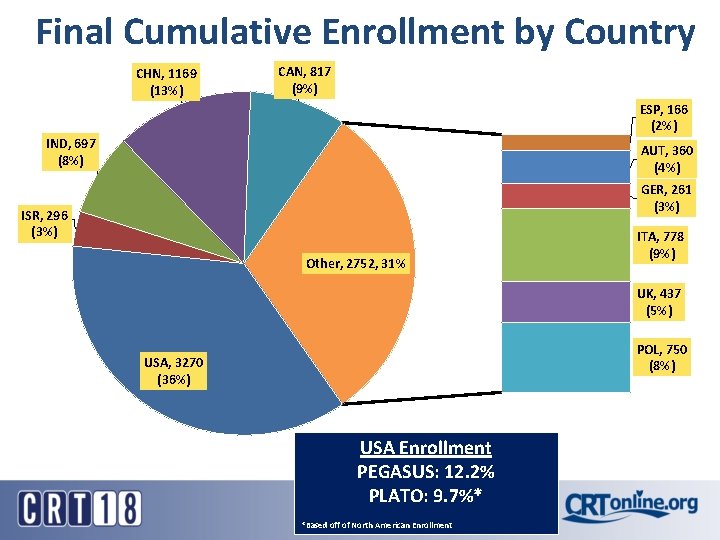
Final Cumulative Enrollment by Country CHN, 1169 (13%) CAN, 817 (9%) ESP, 166 (2%) IND, 697 (8%) AUT, 360 (4%) GER, 261 (3%) ISR, 296 (3%) Other, 2752, 31% ITA, 778 (9%) UK, 437 (5%) POL, 750 (8%) USA, 3270 (36%) USA Enrollment PEGASUS: 12. 2% PLATO: 9. 7%* *Based off of North American Enrollment

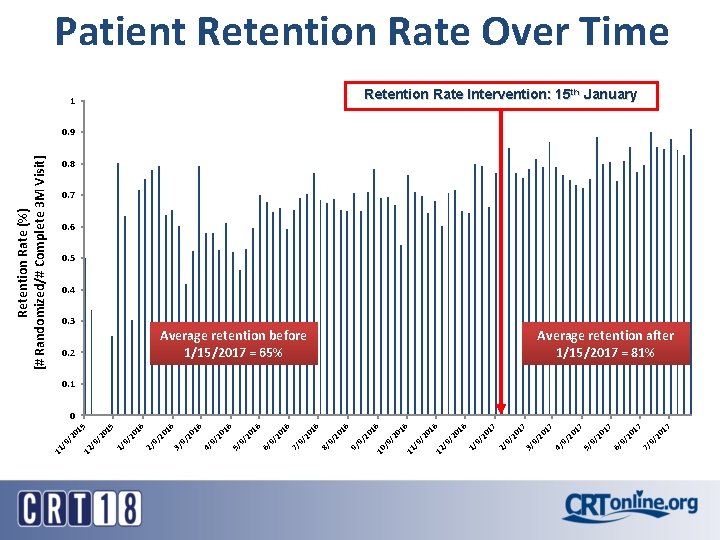
17 17 17 9/ 20 7/ 9/ 20 6/ 17 9/ 20 5/ 9/ 20 17 Average retention before 1/15/2017 = 65% 4/ 9/ 20 3/ 17 17 9/ 20 2/ 16 20 9/ 20 1/ 16 16 20 /9 / 12 /9 / 11 16 20 /9 / 1 10 16 16 9/ 20 9/ 9/ 20 8/ 16 16 9/ 20 7/ 9/ 20 6/ 9/ 20 5/ 16 16 9/ 20 4/ 0. 2 9/ 20 16 0. 3 3/ 15 16 9/ 20 2/ 9/ 20 15 20 /9 / 12 /9 / 11 Retention Rate (%) [# Randomized/# Complete 3 M Visit] Patient Retention Rate Over Time Retention Rate Intervention: 15 th January 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 Average retention after 1/15/2017 = 81% 0. 1 0

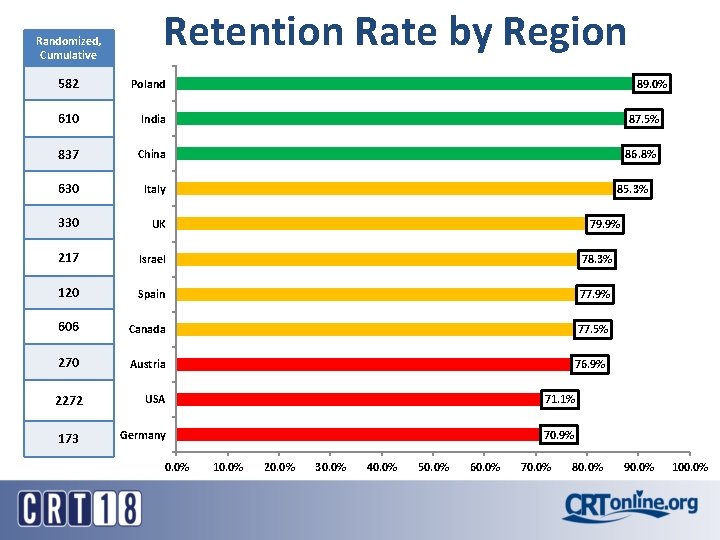
Randomized, Cumulative Retention Rate by Region 582 Poland 610 India 87. 5% 837 China 86. 8% 630 Italy 330 UK 217 Israel 78. 3% 120 Spain 77. 9% 606 Canada 77. 5% 270 Austria 76. 9% 2272 USA 71. 1% 173 Germany 70. 9% 0. 0% 89. 0% 85. 3% 79. 9% 10. 0% 20. 0% 30. 0% 40. 0% 50. 0% 60. 0% 70. 0% 80. 0% 90. 0% 100. 0%

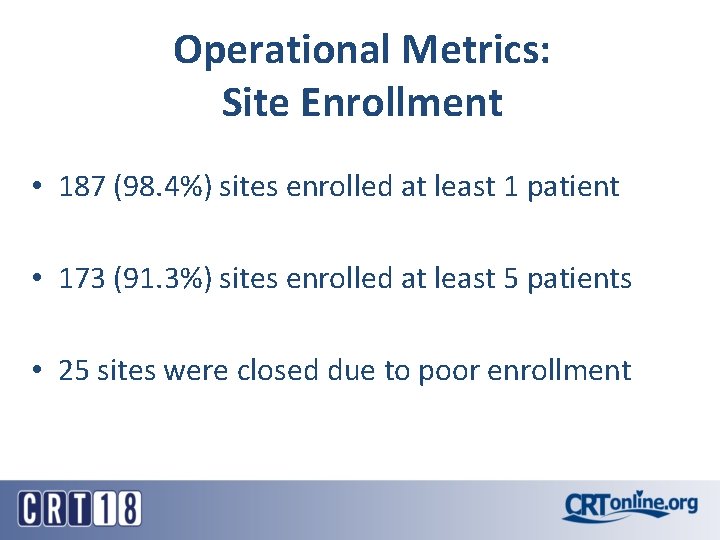
Operational Metrics: Site Enrollment • 187 (98. 4%) sites enrolled at least 1 patient • 173 (91. 3%) sites enrolled at least 5 patients • 25 sites were closed due to poor enrollment

Operational Metrics: Visits Occurring within Follow-up Windows 3 M (Randomization) Visit Occurred Out-of-Window Occurred In-Window 210 3. 1% 6539 96. 9% 238 3. 8% 6015 96. 2% 151 3. 6% 4066 96. 4% 91 4. 9% 1774 95. 1% 54 4. 8% 1082 95. 2% 4 M Visit Occurred Out-of-Window Occurred In-Window 9 M Visit Occurred Out-of-Window Occurred In-Window 15 M Visit Occurred Out-of-Window Occurred In-Window 18 M Visit Occurred Out-of-Window Occurred In-Window

TWILIGHT: Risk Profile Clinical • DM ~ 38% • NSTEMI ~ 28% • Prior Coronary revascularization ~ 50% Procedural • MV PCI ~ 25% • Mod/severe calcium ~ 14% • Stent length >= 30 mm ~ 55%

Future Directions • End of Randomization period: April 2018 • Expected Last Patient Last Visit: April 2019 • Last Patient Contact: June/July 2019
 Classic asp to asp.net migration
Classic asp to asp.net migration Tcagrelor
Tcagrelor Ticagrelor vs prasugrel vs clopidogrel
Ticagrelor vs prasugrel vs clopidogrel Ticagrelor
Ticagrelor Ticagrelor
Ticagrelor Ticagrelor
Ticagrelor Ticagrelor
Ticagrelor Ticagrelor
Ticagrelor Sihd medical abbreviation
Sihd medical abbreviation Ticagrelor
Ticagrelor Asp.net tutorials point
Asp.net tutorials point Asp cordenonese
Asp cordenonese Asp internet
Asp internet Asp.net introduction
Asp.net introduction Asp co to jest
Asp co to jest Syla asp
Syla asp Signe de rovsing
Signe de rovsing Asp bus
Asp bus Fft asp
Fft asp Asp include file
Asp include file