Thymectomy in Nonthymomatous MG Results from MTGX a










- Slides: 31

Thymectomy in Nonthymomatous MG: Results from MTGX, a Randomized, Controlled Trial MSG Scientific Annual Meeting Sept 2016, Snowbird, UT Gil I. Wolfe, MD Dept. of Neurology Univ. at Buffalo/SUNY Jacobs School of Medicine and Biomedical Sciences Buffalo, NY U 01 NS 042685

The Thymectomy Dilemma 1) Why? 2) How? (MGTX trial) 3) What? (MGTX trial results)

The Thymectomy Dilemma 1) Why?

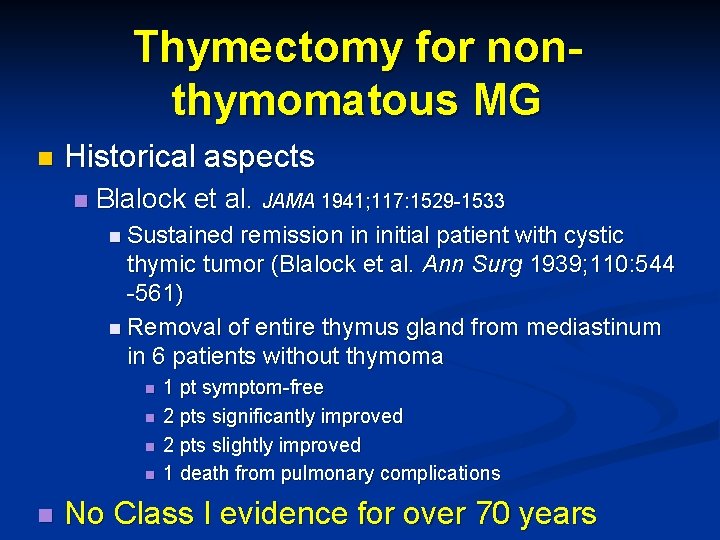
Thymectomy for nonthymomatous MG n Historical aspects n Blalock et al. JAMA 1941; 117: 1529 -1533 n Sustained remission in initial patient with cystic thymic tumor (Blalock et al. Ann Surg 1939; 110: 544 -561) n Removal of entire thymus gland from mediastinum in 6 patients without thymoma n n 1 pt symptom-free 2 pts significantly improved 2 pts slightly improved 1 death from pulmonary complications n No Class I evidence for over 70 years

Thymectomy for MG n Non-thymomatous MG Consensus in favor of thymectomy n Based on non-randomized case series n Repeated calls for randomized, controlled studies n n n Gronseth & Barohn, 2000 (Neurology 55: 7) Grob, 1981 (comments in Ann NY Acad Sci 377: 764) Mc. Quillen & Leone, 1977 (Neurology 27; 1103) Trial efforts failed in 1963, 1980, early 1990 s

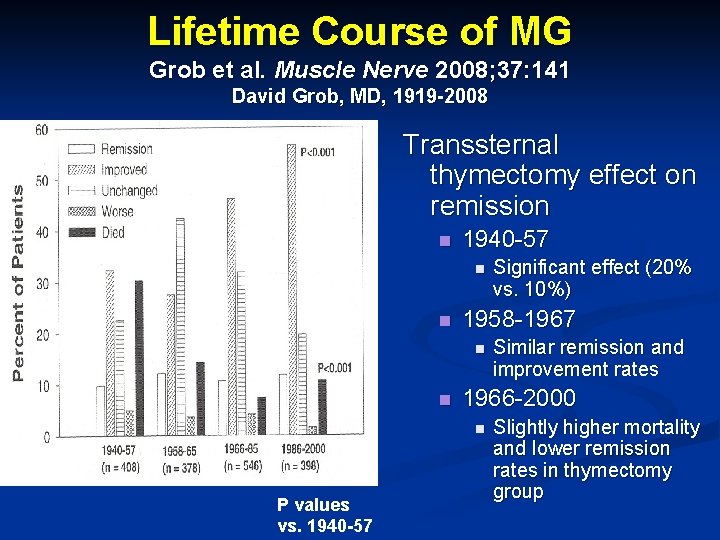
Lifetime Course of MG Grob et al. Muscle Nerve 2008; 37: 141 David Grob, MD, 1919 -2008 Transsternal thymectomy effect on remission n 1940 -57 n n 1958 -1967 n n Similar remission and improvement rates 1966 -2000 n P values vs. 1940 -57 Significant effect (20% vs. 10%) Slightly higher mortality and lower remission rates in thymectomy group

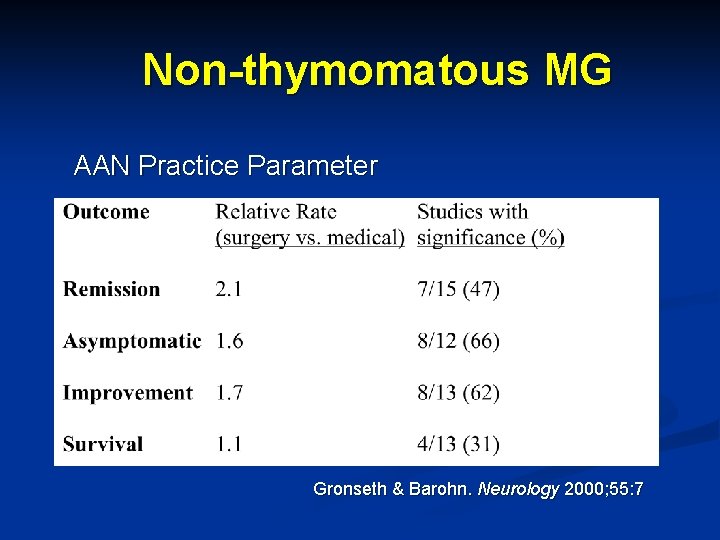
Non-thymomatous MG n Evidence-based AAN Practice Parameter n n n 28 Class II studies (controlled, non-randomized) 21 MG cohorts n n n Gronseth & Barohn. Neurology 2000; 55: 7 4136 surgical pts 4354 non-surgical pts Published between 1953 -1998 Majority used transsternal approach Mean f/u range 3 -28 years No blinded assessments

Non-thymomatous MG AAN Practice Parameter Gronseth & Barohn. Neurology 2000; 55: 7

AAN Practice Parameter n Conclusions and recommendations Benefit of thymectomy not established conclusively n More effective in generalized, severe, female patients? n Recommended as treatment option n Controlled trial needed n n Standardized medical therapy for all arms n Defined outcome measures n Compare different surgical approaches n Gronseth & Barohn. Neurology 2000; 55: 7

The Thymectomy Dilemma 1) Why? 2) How?

MGTX and Bio. MG Executive Committee John Newsom-Davis, MD (Study Chair, emeritus) Gary Cutter, Ph. D (Director, DCC, UAB) Gil Wolfe, MD (Study Chair) Henry Kaminski, MD (Study Vice Chair; Director Bio. MG) Inmaculada Aban, Ph. D (Deputy Director, DCC) Alfred Jaretzki, MD (Surgical Chair, emeritus) Joshua Sonnett, MD (Surgical Chair) Greg Minisman (Project Manager) Robin Conwit, MD (NINDS Program Director) Joanne Odenkirchen, MPH (NINDS colleague) U 01 NS 042685 1919 -2014 1932 -2007

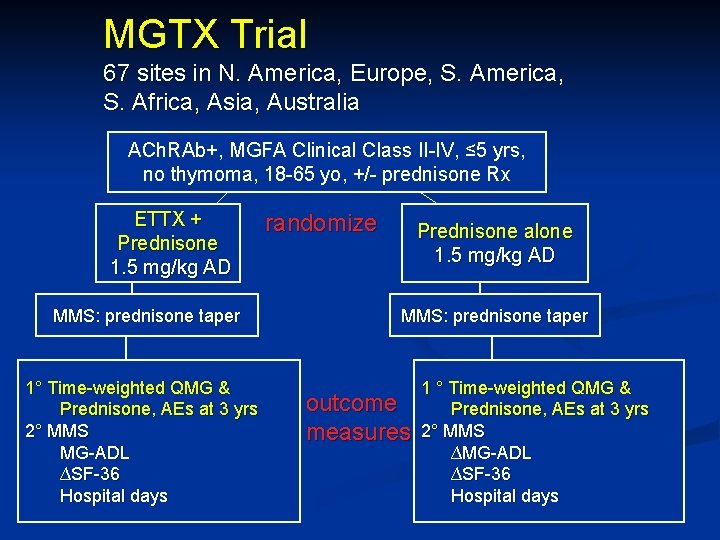
MGTX: Single blind, Multicenter, International Randomized Trial Primary aim: Answer 3 questions in nonthymomatous MG patients followed for 3 years Compared with prednisone protocol alone, does extended transsternal thymectomy (ETTX) + prednisone protocol result in: • greater improvement in strength (time-weighted average QMG)? • lower prednisone requirement (time-weighted average prednisone dose)? • enhanced quality of life (AEs, TAS, TAC)?

MGTX protocol details Inclusion criteria ACh. R binding Ab pos (≥ 0. 5) MGFA Class 2 -4; disease duration < 5 years Age at least 18 and < 65 years Optimal anti-cholinesterase dose Prednisone naive or not Main exclusion criteria Previous thymectomy or sternotomy or thoracotomy Immunosuppressive therapy (x prednisone) within last year Rituximab at any time Medically or psychiatrically unfit for thymectomy Chest CT or MR evidence of thymoma Pregnancy or lactation, or considering becoming pregnant Current prednisone > 0. 75 mg/kg or 50 mg/d (or AD equivalent)

MGTX Trial 67 sites in N. America, Europe, S. America, S. Africa, Asia, Australia ACh. RAb+, MGFA Clinical Class II-IV, ≤ 5 yrs, no thymoma, 18 -65 yo, +/- prednisone Rx ETTX + Prednisone 1. 5 mg/kg AD MMS: prednisone taper 1° Time-weighted QMG & Prednisone, AEs at 3 yrs 2° MMS MG-ADL ∆SF-36 Hospital days randomize Prednisone alone 1. 5 mg/kg AD MMS: prednisone taper outcome measures 1 ° Time-weighted QMG & Prednisone, AEs at 3 yrs 2° MMS ∆MG-ADL ∆SF-36 Hospital days

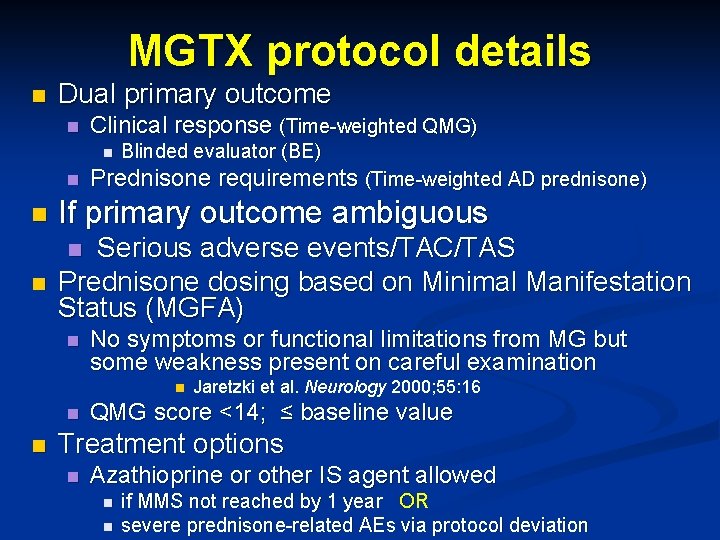
MGTX protocol details n Dual primary outcome n Clinical response (Time-weighted QMG) n n Blinded evaluator (BE) Prednisone requirements (Time-weighted AD prednisone) n If primary outcome ambiguous n Serious adverse events/TAC/TAS Prednisone dosing based on Minimal Manifestation Status (MGFA) n n No symptoms or functional limitations from MG but some weakness present on careful examination n Jaretzki et al. Neurology 2000; 55: 16 QMG score <14; ≤ baseline value Treatment options n Azathioprine or other IS agent allowed n n if MMS not reached by 1 year OR severe prednisone-related AEs via protocol deviation

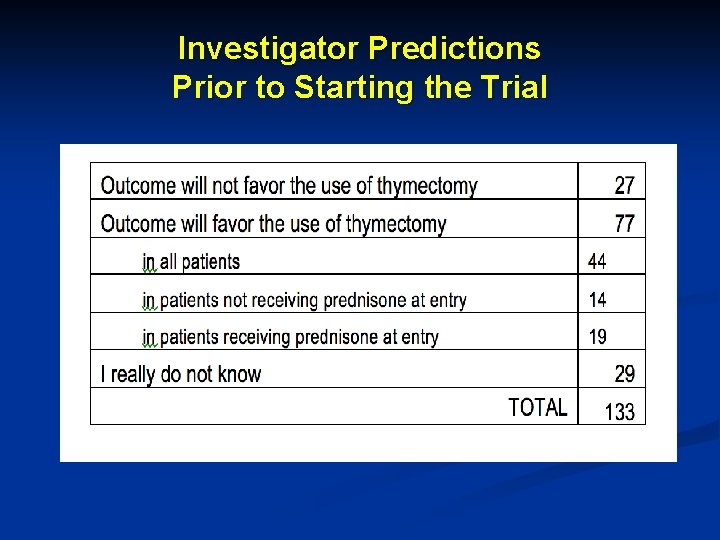
Investigator Predictions Prior to Starting the Trial

MGTX Results 1) Why? 2) How? 3) What? Wolfe GI et al. N Engl J Med 2016; 375: 511 -522

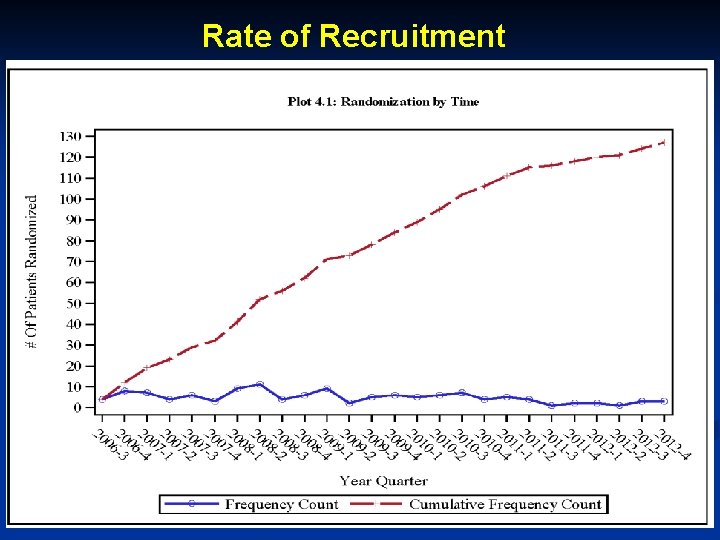
Rate of Recruitment 18

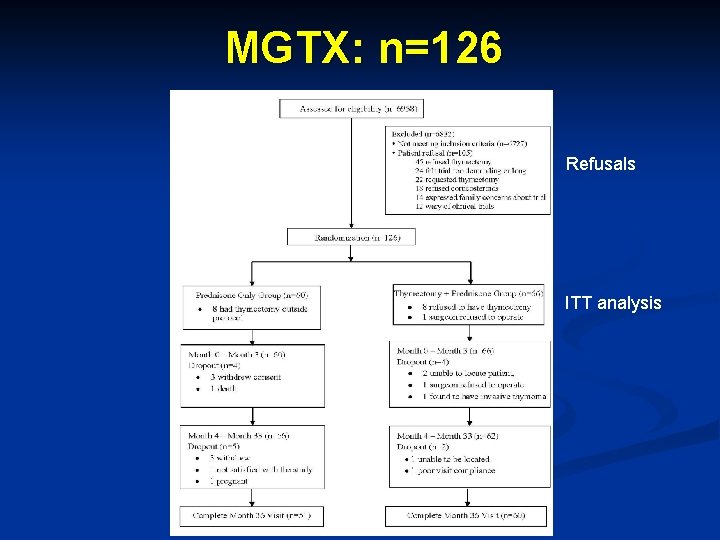
MGTX: n=126 Refusals ITT analysis

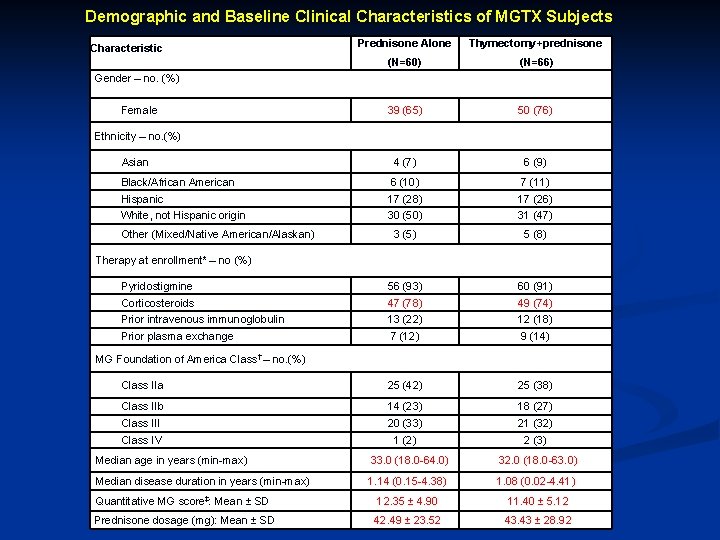
Demographic and Baseline Clinical Characteristics of MGTX Subjects Characteristic Prednisone Alone Thymectomy+prednisone (N=60) (N=66) 39 (65) 50 (76) Asian 4 (7) 6 (9) Black/African American 6 (10) 7 (11) Hispanic 17 (28) 17 (26) White, not Hispanic origin 30 (50) 31 (47) 3 (5) 5 (8) Pyridostigmine 56 (93) 60 (91) Corticosteroids 47 (78) 49 (74) Prior intravenous immunoglobulin 13 (22) 12 (18) Prior plasma exchange 7 (12) 9 (14) Class IIa 25 (42) 25 (38) Class IIb 14 (23) 18 (27) Class III 20 (33) 21 (32) Class IV 1 (2) 2 (3) Gender – no. (%) Female Ethnicity – no. (%) Other (Mixed/Native American/Alaskan) Therapy at enrollment* – no (%) MG Foundation of America Class† – no. (%) Median age in years (min-max) Median disease duration in years (min-max) Quantitative MG score‡: Mean ± SD Prednisone dosage (mg): Mean ± SD 33. 0 (18. 0 -64. 0) 32. 0 (18. 0 -63. 0) 1. 14 (0. 15 -4. 38) 1. 08 (0. 02 -4. 41) 12. 35 ± 4. 90 11. 40 ± 5. 12 42. 49 ± 23. 52 43. 43 ± 28. 92

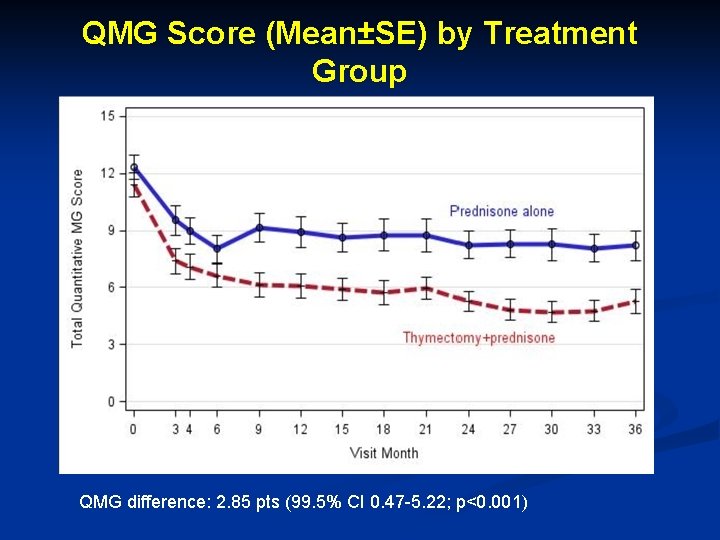
QMG Score (Mean±SE) by Treatment Group QMG difference: 2. 85 pts (99. 5% CI 0. 47 -5. 22; p<0. 001)

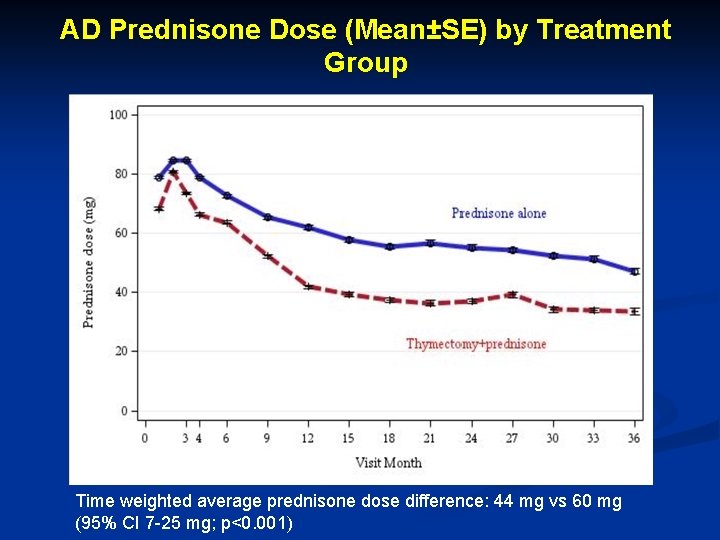
AD Prednisone Dose (Mean±SE) by Treatment Group Time weighted average prednisone dose difference: 44 mg vs 60 mg (95% CI 7 -25 mg; p<0. 001)

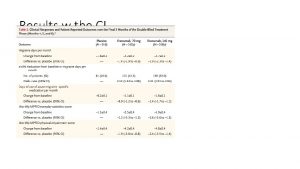
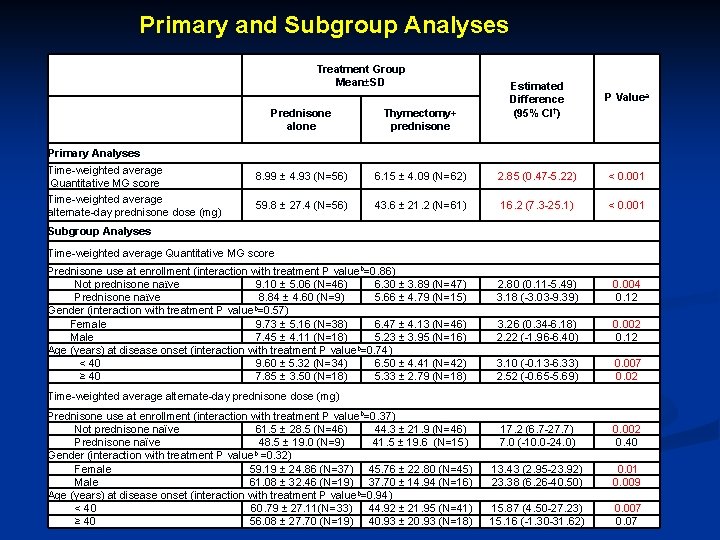
Primary and Subgroup Analyses Treatment Group Mean±SD Estimated Difference (95% CI†) P Valuea Prednisone alone Thymectomy+ prednisone 8. 99 ± 4. 93 (N=56) 6. 15 ± 4. 09 (N=62) 2. 85 (0. 47 -5. 22) < 0. 001 59. 8 ± 27. 4 (N=56) 43. 6 ± 21. 2 (N=61) 16. 2 (7. 3 -25. 1) < 0. 001 2. 80 (0. 11 -5. 49) 3. 18 (-3. 03 -9. 39) 0. 004 0. 12 3. 26 (0. 34 -6. 18) 2. 22 (-1. 96 -6. 40) 0. 002 0. 12 3. 10 (-0. 13 -6. 33) 2. 52 (-0. 65 -5. 69) 0. 007 0. 02 17. 2 (6. 7 -27. 7) 7. 0 (-10. 0 -24. 0) 0. 002 0. 40 13. 43 (2. 95 -23. 92) 23. 38 (6. 26 -40. 50) 0. 01 0. 009 15. 87 (4. 50 -27. 23) 15. 16 (-1. 30 -31. 62) 0. 007 0. 07 Primary Analyses Time-weighted average Quantitative MG score Time-weighted average alternate-day prednisone dose (mg) Subgroup Analyses Time-weighted average Quantitative MG score Prednisone use at enrollment (interaction with treatment P valueb=0. 86) Not prednisone naїve 9. 10 ± 5. 06 (N=46) 6. 30 ± 3. 89 (N=47) Prednisone naїve 8. 84 ± 4. 60 (N=9) 5. 66 ± 4. 79 (N=15) Gender (interaction with treatment P valueb=0. 57) Female 9. 73 ± 5. 16 (N=38) 6. 47 ± 4. 13 (N=46) Male 7. 45 ± 4. 11 (N=18) 5. 23 ± 3. 95 (N=16) Age (years) at disease onset (interaction with treatment P valueb=0. 74) < 40 9. 60 ± 5. 32 (N=34) 6. 50 ± 4. 41 (N=42) ≥ 40 7. 85 ± 3. 50 (N=18) 5. 33 ± 2. 79 (N=18) Time-weighted average alternate-day prednisone dose (mg) Prednisone use at enrollment (interaction with treatment P valueb=0. 37) Not prednisone naїve 61. 5 ± 28. 5 (N=46) 44. 3 ± 21. 9 (N=46) Prednisone naїve 48. 5 ± 19. 0 (N=9) 41. 5 ± 19. 6 (N=15) Gender (interaction with treatment P valueb =0. 32) Female 59. 19 ± 24. 86 (N=37) 45. 76 ± 22. 80 (N=45) Male 61. 08 ± 32. 46 (N=19) 37. 70 ± 14. 94 (N=16) Age (years) at disease onset (interaction with treatment P valueb=0. 94) < 40 60. 79 ± 27. 11(N=33) 44. 92 ± 21. 95 (N=41) ≥ 40 56. 08 ± 27. 70 (N=19) 40. 93 ± 20. 93 (N=18)

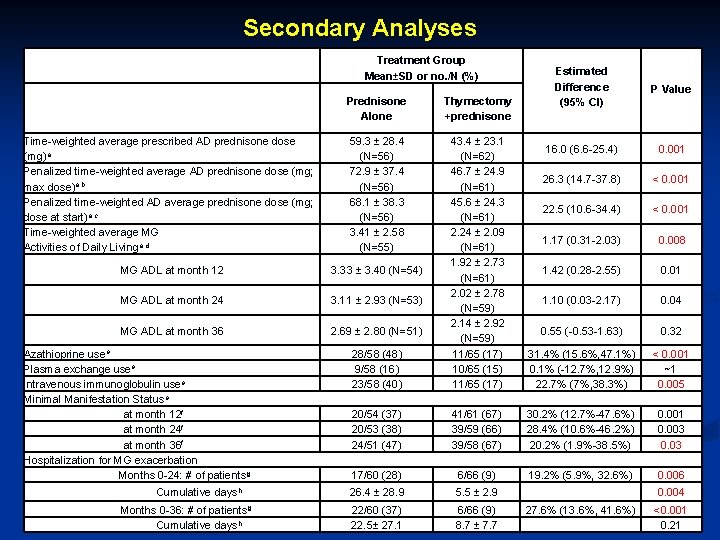
Secondary Analyses Treatment Group Mean±SD or no. /N (%) Estimated Difference (95% CI) P Value 16. 0 (6. 6 -25. 4) 0. 001 26. 3 (14. 7 -37. 8) < 0. 001 22. 5 (10. 6 -34. 4) < 0. 001 1. 17 (0. 31 -2. 03) 0. 008 1. 42 (0. 28 -2. 55) 0. 01 1. 10 (0. 03 -2. 17) 0. 04 Prednisone Alone Thymectomy +prednisone Time-weighted average prescribed AD prednisone dose (mg)a Penalized time-weighted average AD prednisone dose (mg; max dose)a, b Penalized time-weighted AD average prednisone dose (mg; dose at start)a, c Time-weighted average MG Activities of Daily Livinga, d 59. 3 ± 28. 4 (N=56) 72. 9 ± 37. 4 (N=56) 68. 1 ± 38. 3 (N=56) 3. 41 ± 2. 58 (N=55) MG ADL at month 12 3. 33 ± 3. 40 (N=54) MG ADL at month 24 3. 11 ± 2. 93 (N=53) MG ADL at month 36 2. 69 ± 2. 80 (N=51) 0. 55 (-0. 53 -1. 63) 0. 32 28/58 (48) 9/58 (16) 23/58 (40) 43. 4 ± 23. 1 (N=62) 46. 7 ± 24. 9 (N=61) 45. 6 ± 24. 3 (N=61) 2. 24 ± 2. 09 (N=61) 1. 92 ± 2. 73 (N=61) 2. 02 ± 2. 78 (N=59) 2. 14 ± 2. 92 (N=59) 11/65 (17) 10/65 (15) 11/65 (17) 31. 4% (15. 6%, 47. 1%) 0. 1% (-12. 7%, 12. 9%) 22. 7% (7%, 38. 3%) < 0. 001 ~1 0. 005 20/54 (37) 20/53 (38) 24/51 (47) 41/61 (67) 39/59 (66) 39/58 (67) 30. 2% (12. 7%-47. 6%) 28. 4% (10. 6%-46. 2%) 20. 2% (1. 9%-38. 5%) 0. 001 0. 003 0. 03 17/60 (28) 6/66 (9) 19. 2% (5. 9%, 32. 6%) 0. 006 26. 4 ± 28. 9 5. 5 ± 2. 9 0. 004 22/60 (37) 22. 5± 27. 1 6/66 (9) 8. 7 ± 7. 7 27. 6% (13. 6%, 41. 6%) <0. 001 0. 21 Azathioprine usee Plasma exchange usee Intravenous immunoglobulin usee Minimal Manifestation Statuse at month 12 f at month 24 f at month 36 f Hospitalization for MG exacerbation Months 0 -24: # of patientsg Cumulative daysh Months 0 -36: # of patientsg Cumulative daysh

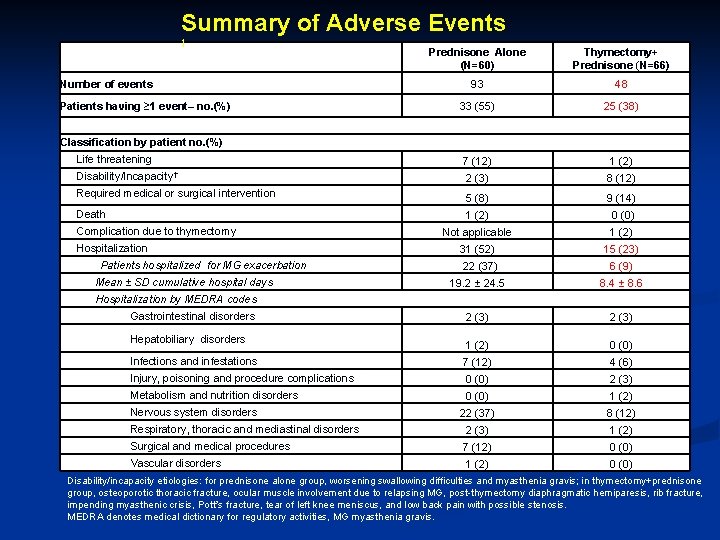
Summary of Adverse Events † Prednisone Alone (N=60) Thymectomy+ Prednisone (N=66) 93 48 33 (55) 25 (38) Life threatening 7 (12) 1 (2) Disability/Incapacity† 2 (3) 8 (12) 5 (8) 9 (14) 1 (2) 0 (0) Not applicable 1 (2) 31 (52) 15 (23) 22 (37) 6 (9) 19. 2 ± 24. 5 8. 4 ± 8. 6 2 (3) 1 (2) 0 (0) Infections and infestations 7 (12) 4 (6) Injury, poisoning and procedure complications 0 (0) 2 (3) Metabolism and nutrition disorders 0 (0) 1 (2) 22 (37) 8 (12) Respiratory, thoracic and mediastinal disorders 2 (3) 1 (2) Surgical and medical procedures 7 (12) 0 (0) Vascular disorders 1 (2) 0 (0) Number of events Patients having ≥ 1 event– no. (%) Classification by patient no. (%) Required medical or surgical intervention Death Complication due to thymectomy Hospitalization Patients hospitalized for MG exacerbation Mean ± SD cumulative hospital days Hospitalization by MEDRA codes Gastrointestinal disorders Hepatobiliary disorders Nervous system disorders Disability/incapacity etiologies: for prednisone alone group, worsening swallowing difficulties and myasthenia gravis; in thymectomy+prednisone group, osteoporotic thoracic fracture, ocular muscle involvement due to relapsing MG, post-thymectomy diaphragmatic hemiparesis, rib fracture, impending myasthenic crisis, Pott’s fracture, tear of left knee meniscus, and low back pain with possible stenosis. MEDRA denotes medical dictionary for regulatory activities, MG myasthenia gravis.

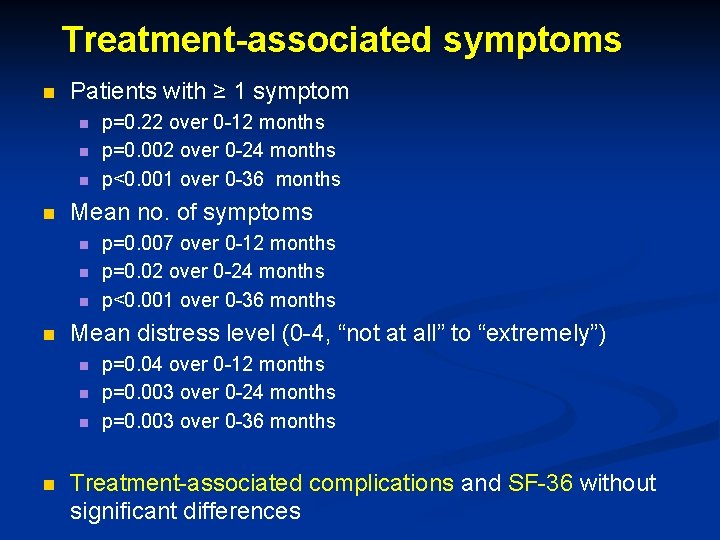
Treatment-associated symptoms n Patients with ≥ 1 symptom n n Mean no. of symptoms n n p=0. 007 over 0 -12 months p=0. 02 over 0 -24 months p<0. 001 over 0 -36 months Mean distress level (0 -4, “not at all” to “extremely”) n n p=0. 22 over 0 -12 months p=0. 002 over 0 -24 months p<0. 001 over 0 -36 months p=0. 04 over 0 -12 months p=0. 003 over 0 -24 months p=0. 003 over 0 -36 months Treatment-associated complications and SF-36 without significant differences

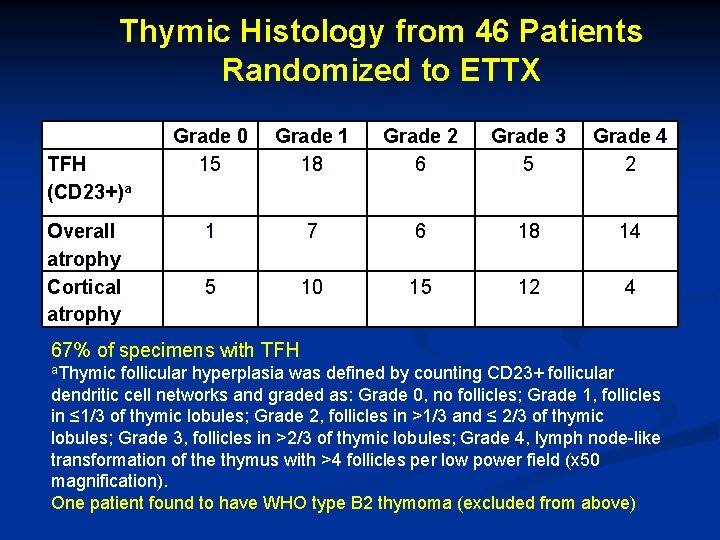
Thymic Histology from 46 Patients Randomized to ETTX TFH (CD 23+)a Grade 0 15 Grade 1 18 Grade 2 6 Grade 3 5 Grade 4 2 Overall atrophy Cortical atrophy 1 7 6 18 14 5 10 15 12 4 67% of specimens with TFH a. Thymic follicular hyperplasia was defined by counting CD 23+ follicular dendritic cell networks and graded as: Grade 0, no follicles; Grade 1, follicles in ≤ 1/3 of thymic lobules; Grade 2, follicles in >1/3 and ≤ 2/3 of thymic lobules; Grade 3, follicles in >2/3 of thymic lobules; Grade 4, lymph node-like transformation of the thymus with >4 follicles per low power field (x 50 magnification). One patient found to have WHO type B 2 thymoma (excluded from above)

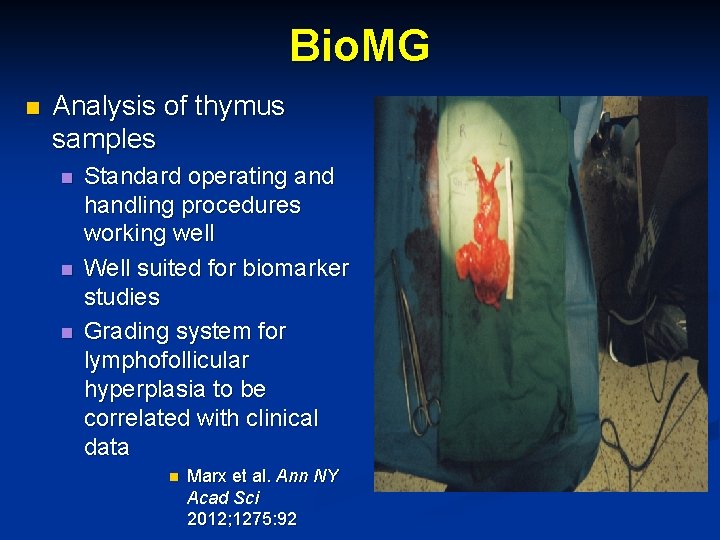
Bio. MG n Analysis of thymus samples n n n Standard operating and handling procedures working well Well suited for biomarker studies Grading system for lymphofollicular hyperplasia to be correlated with clinical data n Marx et al. Ann NY Acad Sci 2012; 1275: 92

MGTX in Context Statements for non-thymomatous MG n Cochrane Collaboration n “There is no randomized controlled trial literature that allows meaningful conclusions about the efficacy of thymectomy on MG. Data from several class III observational studies suggest that thymectomy could be beneficial in MG. An RCT is needed…” n n Cea G, et al. Cochrane Library 2013; 10: 1 -20 Intl MG Treatment Recommendations n “In non-thymomatous MG, thymectomy is performed as an option to potentially avoid or minimize the dose or duration of immunotherapy, or if patients fail to respond to an initial trial of immunotherapy or have intolerable side effects from that therapy. ” n Sanders DB, Wolfe GI, Benatar M, et al. Neurology 2016; 87: 419 -425

MGTX Summary n Class I evidence: ETTX has favorable impact on ACh. RAb+ generalized MG Clinical outcomes n Corticosteroid exposure n Adverse events n Effect can be seen in <12 months n Future investigations n Predictive role of thymic histology n Bio. MG: Genomic/proteomic profiles, biomarker assessments n Wolfe GI et al. N Engl J Med 2016; 375: 511 -522

Many Thanks! n n Study subjects: Trust in randomization; ≥ 3 yrs MGTX Investigators/DSMB March 2006 San Fran / Oxford
 Thymectomy
Thymectomy Myasthenia gravis thymectomy
Myasthenia gravis thymectomy 17 testo methox
17 testo methox Nzqa digital exams results
Nzqa digital exams results Labfinder/paradigm results
Labfinder/paradigm results Oxidase results
Oxidase results Kligler iron agar results
Kligler iron agar results Drdp summary of findings
Drdp summary of findings Rso test of generator rotor
Rso test of generator rotor Multiplying or dividing vectors by scalars results in:
Multiplying or dividing vectors by scalars results in: Brigance scoring chart 5 year old
Brigance scoring chart 5 year old Costophrenic angle
Costophrenic angle Who fought in punic wars
Who fought in punic wars Starch hydrolysis positive result
Starch hydrolysis positive result 5 love languages test
5 love languages test Www.ielts.org results
Www.ielts.org results Liberia census 2008 results
Liberia census 2008 results Hp fortify scan
Hp fortify scan Asch conformity experiment results
Asch conformity experiment results Rws sdsu
Rws sdsu Valid and invalid arguments examples
Valid and invalid arguments examples Nt scan results chart
Nt scan results chart Write a result
Write a result Wwwcxc
Wwwcxc Vincent minier
Vincent minier Fdot procurement office
Fdot procurement office Chromosomal nondisjunction results in
Chromosomal nondisjunction results in Measures of variability range
Measures of variability range Cxc results 2018
Cxc results 2018 Cfa level 1 learning ecosystem
Cfa level 1 learning ecosystem Forthcoming results
Forthcoming results Oas imu
Oas imu