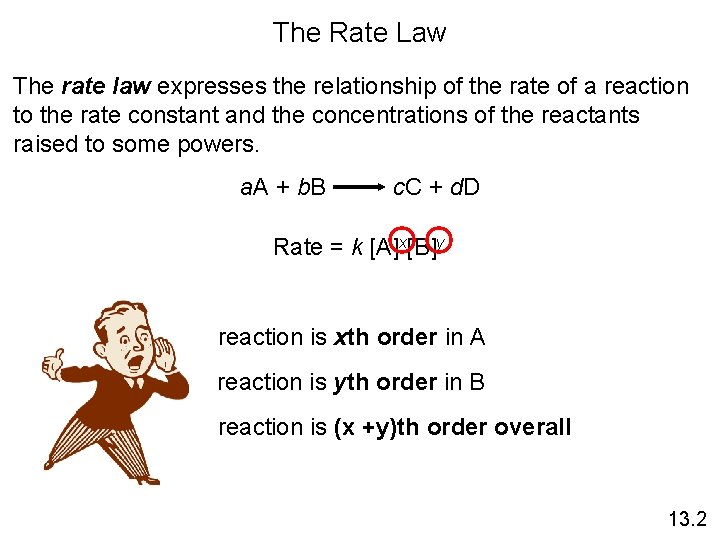
The Rate Law The rate law expresses the
![First-Order Reactions A k= product D[A] rate = Dt rate M/s = = 1/s First-Order Reactions A k= product D[A] rate = Dt rate M/s = = 1/s](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-5.jpg)
![Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M • Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M •](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-8.jpg)
![Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt rate = M/s Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt rate = M/s](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-9.jpg)
![Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-16.jpg)
- Slides: 20

The Rate Law The rate law expresses the relationship of the rate of a reaction to the rate constant and the concentrations of the reactants raised to some powers. a. A + b. B c. C + d. D Rate = k [A]x[B]y reaction is xth order in A reaction is yth order in B reaction is (x +y)th order overall 13. 2

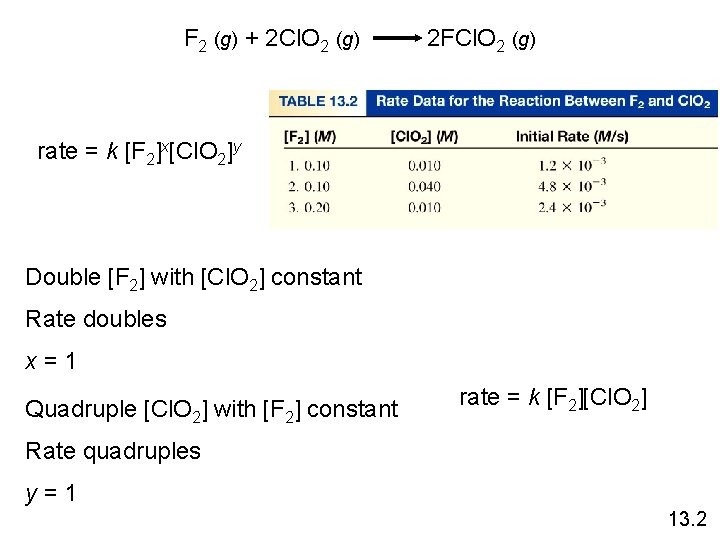
F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2]x[Cl. O 2]y Double [F 2] with [Cl. O 2] constant Rate doubles x=1 Quadruple [Cl. O 2] with [F 2] constant rate = k [F 2][Cl. O 2] Rate quadruples y=1 13. 2

Rate Laws • Rate laws are always determined experimentally. • Reaction order is always defined in terms of reactant (not product) concentrations. • The order of a reactant is not related to the stoichiometric coefficient of the reactant in the balanced chemical equation. F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2][Cl. O 2] 1 13. 2

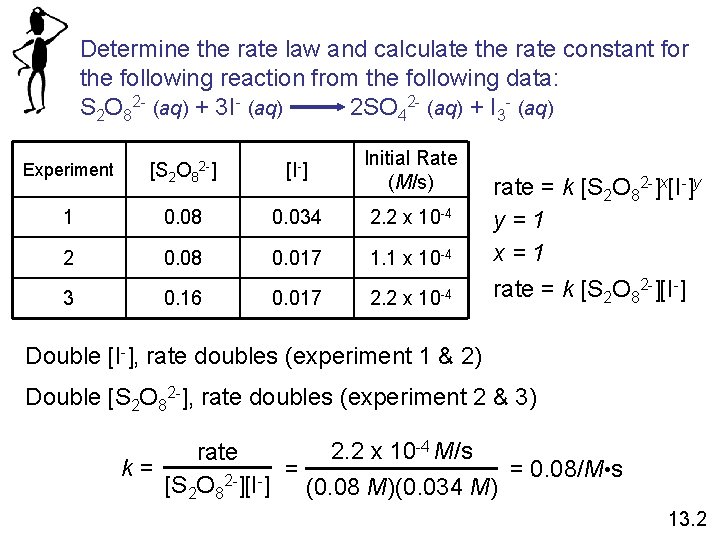
Determine the rate law and calculate the rate constant for the following reaction from the following data: S 2 O 82 - (aq) + 3 I- (aq) 2 SO 42 - (aq) + I 3 - (aq) Experiment [S 2 O 82 -] [I-] Initial Rate (M/s) 1 0. 08 0. 034 2. 2 x 10 -4 2 0. 08 0. 017 1. 1 x 10 -4 3 0. 16 0. 017 2. 2 x 10 -4 rate = k [S 2 O 82 -]x[I-]y y=1 x=1 rate = k [S 2 O 82 -][I-] Double [I-], rate doubles (experiment 1 & 2) Double [S 2 O 82 -], rate doubles (experiment 2 & 3) 2. 2 x 10 -4 M/s rate k= = = 0. 08/M • s 2[S 2 O 8 ][I ] (0. 08 M)(0. 034 M) 13. 2
![FirstOrder Reactions A k product DA rate Dt rate Ms 1s First-Order Reactions A k= product D[A] rate = Dt rate M/s = = 1/s](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-5.jpg)
First-Order Reactions A k= product D[A] rate = Dt rate M/s = = 1/s or s-1 M [A] = [A]0 exp(-kt) rate = k [A] D[A] = k [A] Dt [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 ln[A] = ln[A]0 - kt 13. 3

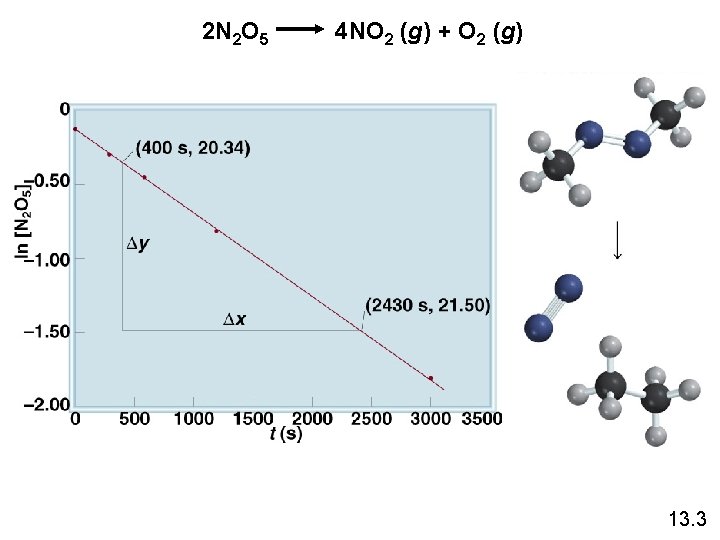
2 N 2 O 5 4 NO 2 (g) + O 2 (g) 13. 3

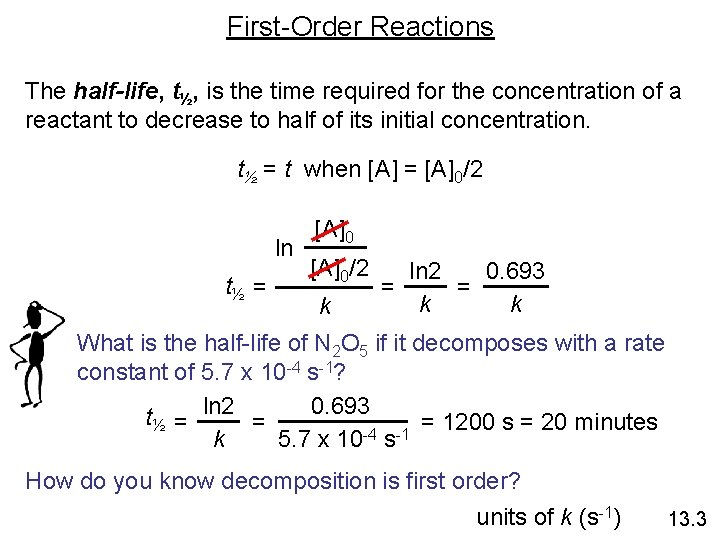
First-Order Reactions The half-life, t½, is the time required for the concentration of a reactant to decrease to half of its initial concentration. t½ = t when [A] = [A]0/2 ln t½ = [A]0/2 k ln 2 0. 693 = = k k What is the half-life of N 2 O 5 if it decomposes with a rate constant of 5. 7 x 10 -4 s-1? 0. 693 t½ = ln 2 = = 1200 s = 20 minutes -4 -1 k 5. 7 x 10 s How do you know decomposition is first order? units of k (s-1) 13. 3
![SecondOrder Reactions A product DA rate Dt rate Ms 1M Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M •](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-8.jpg)
Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M • s k= 2 2 M [A] 1 1 = + kt [A]0 rate = k [A]2 D[A] = k [A]2 Dt [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 t½ = t when [A] = [A]0/2 1 t½ = k[A]0 13. 3
![ZeroOrder Reactions A product DA rate Dt DA k Dt rate Ms Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt rate = M/s](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-9.jpg)
Zero-Order Reactions A product D[A] rate = Dt D[A] =k Dt rate = M/s k= 0 [A] = [A]0 - kt rate = k [A]0 = k [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 t½ = t when [A] = [A]0/2 [A]0 t½ = 2 k 13. 3

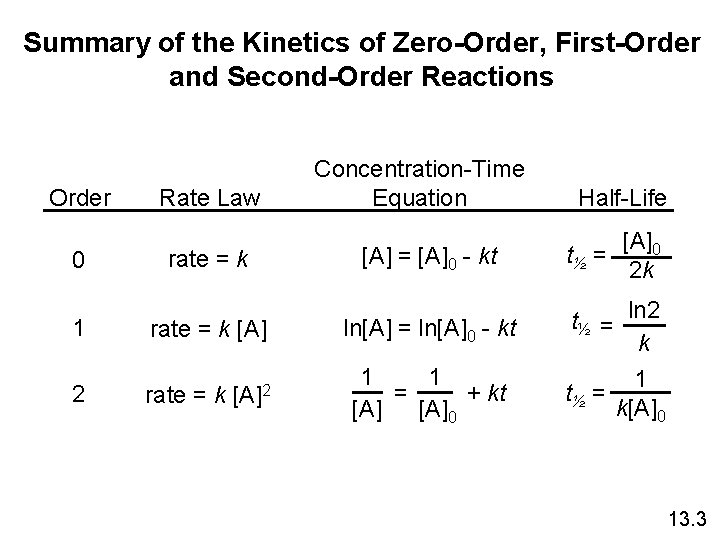
Summary of the Kinetics of Zero-Order, First-Order and Second-Order Reactions Order 0 Rate Law rate = k 1 rate = k [A] 2 [A]2 rate = k Concentration-Time Equation [A] = [A]0 - kt Half-Life t½ = [A]0 2 k ln[A] = ln[A]0 - kt t½ = ln 2 k 1 1 = + kt [A]0 1 t½ = k[A]0 13. 3

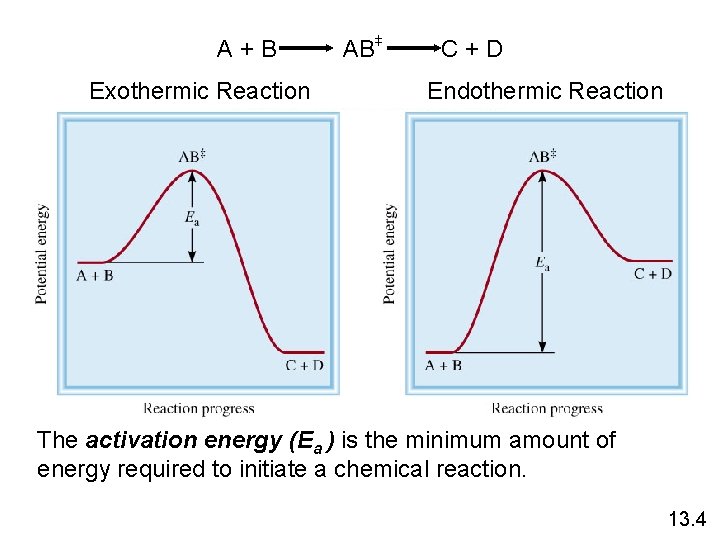
A+B Exothermic Reaction + + AB C+D Endothermic Reaction The activation energy (Ea ) is the minimum amount of energy required to initiate a chemical reaction. 13. 4

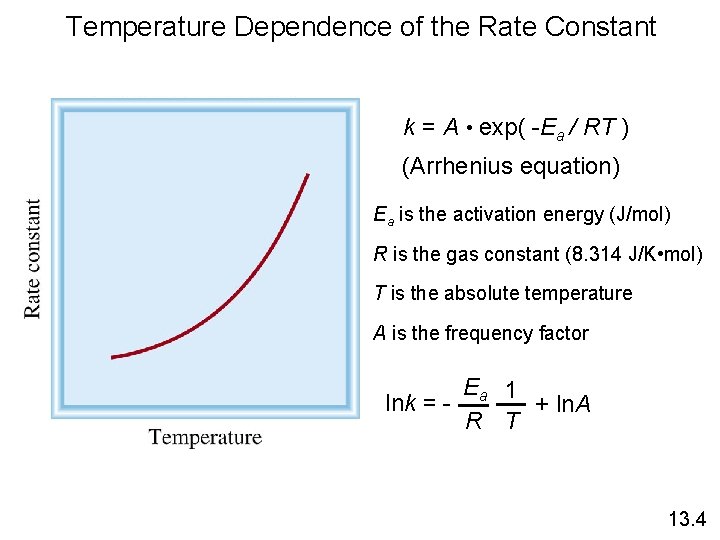
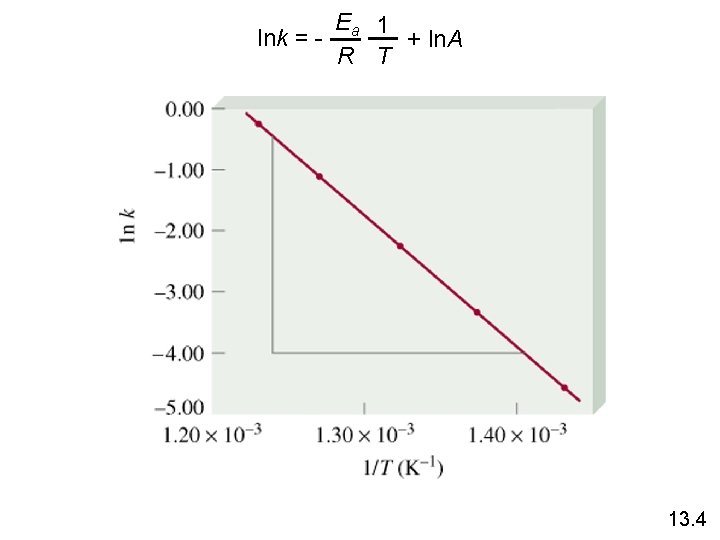
Temperature Dependence of the Rate Constant k = A • exp( -Ea / RT ) (Arrhenius equation) Ea is the activation energy (J/mol) R is the gas constant (8. 314 J/K • mol) T is the absolute temperature A is the frequency factor Ea 1 lnk = + ln. A R T 13. 4

Ea 1 lnk = + ln. A R T 13. 4

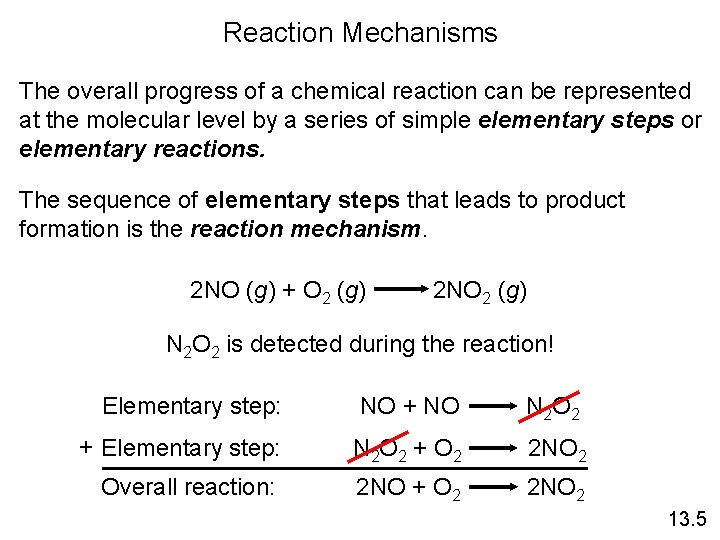
Reaction Mechanisms The overall progress of a chemical reaction can be represented at the molecular level by a series of simple elementary steps or elementary reactions. The sequence of elementary steps that leads to product formation is the reaction mechanism. 2 NO (g) + O 2 (g) 2 NO 2 (g) N 2 O 2 is detected during the reaction! Elementary step: NO + NO N 2 O 2 + Elementary step: N 2 O 2 + O 2 2 NO 2 Overall reaction: 2 NO + O 2 2 NO 2 13. 5

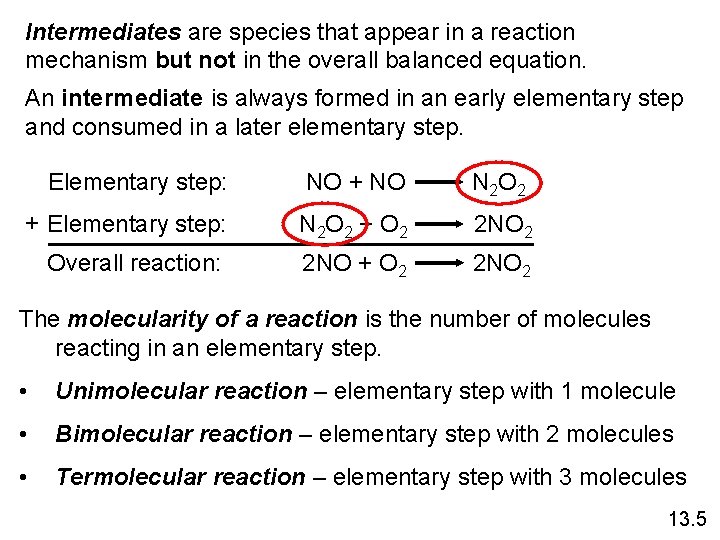
Intermediates are species that appear in a reaction mechanism but not in the overall balanced equation. An intermediate is always formed in an early elementary step and consumed in a later elementary step. Elementary step: NO + NO N 2 O 2 + Elementary step: N 2 O 2 + O 2 2 NO 2 Overall reaction: 2 NO + O 2 2 NO 2 The molecularity of a reaction is the number of molecules reacting in an elementary step. • Unimolecular reaction – elementary step with 1 molecule • Bimolecular reaction – elementary step with 2 molecules • Termolecular reaction – elementary step with 3 molecules 13. 5
![Rate Laws and Elementary Steps Unimolecular reaction A products rate k A Bimolecular Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular](https://slidetodoc.com/presentation_image_h2/ec74f80983480c50be58e124339dc7eb/image-16.jpg)
Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular reaction A+B products rate = k [A][B] Bimolecular reaction A+A products rate = k [A]2 Writing plausible reaction mechanisms: • The sum of the elementary steps must give the overall balanced equation for the reaction. • The rate-determining step should predict the same rate law that is determined experimentally. The rate-determining step is the slowest step in the sequence of steps leading to product formation. 13. 5

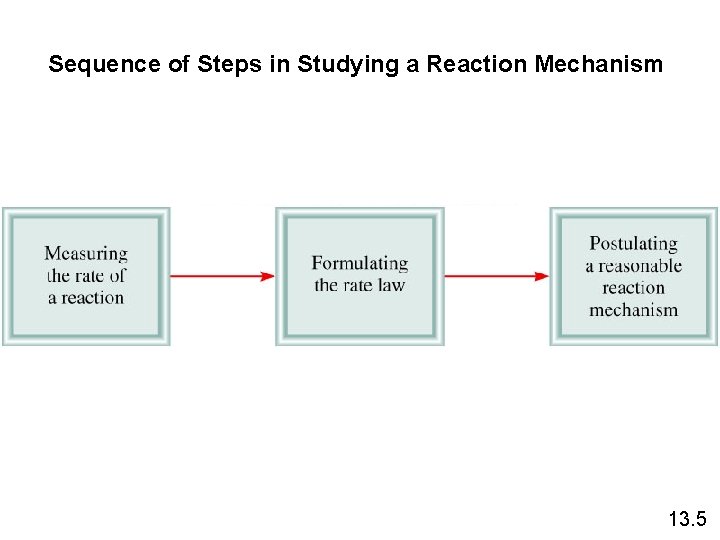
Sequence of Steps in Studying a Reaction Mechanism 13. 5

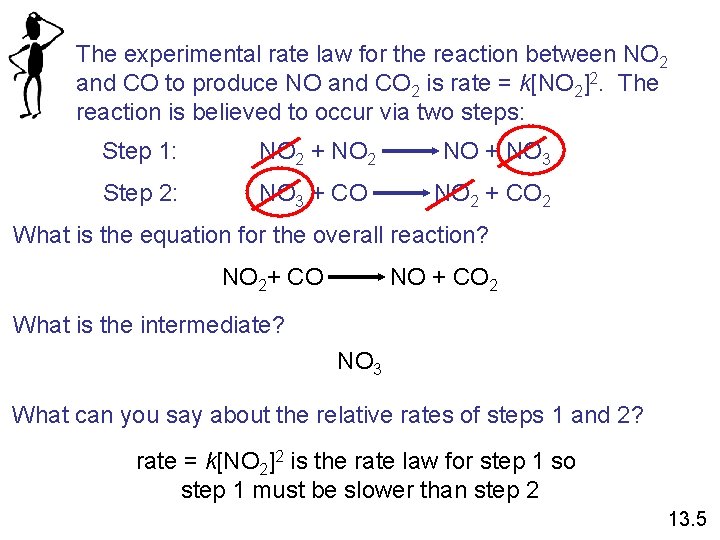
The experimental rate law for the reaction between NO 2 and CO to produce NO and CO 2 is rate = k[NO 2]2. The reaction is believed to occur via two steps: Step 1: NO 2 + NO 2 NO + NO 3 Step 2: NO 3 + CO NO 2 + CO 2 What is the equation for the overall reaction? NO 2+ CO NO + CO 2 What is the intermediate? NO 3 What can you say about the relative rates of steps 1 and 2? rate = k[NO 2]2 is the rate law for step 1 so step 1 must be slower than step 2 13. 5

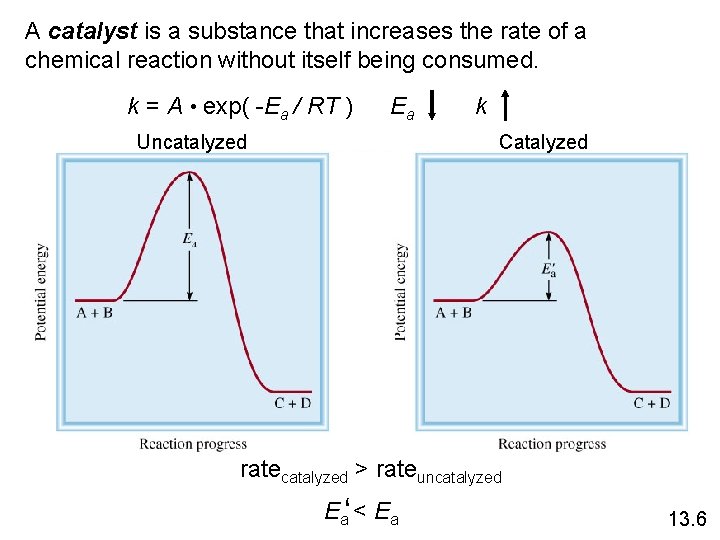
A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed. k = A • exp( -Ea / RT ) Ea Uncatalyzed k Catalyzed ratecatalyzed > rateuncatalyzed Ea‘ < Ea 13. 6

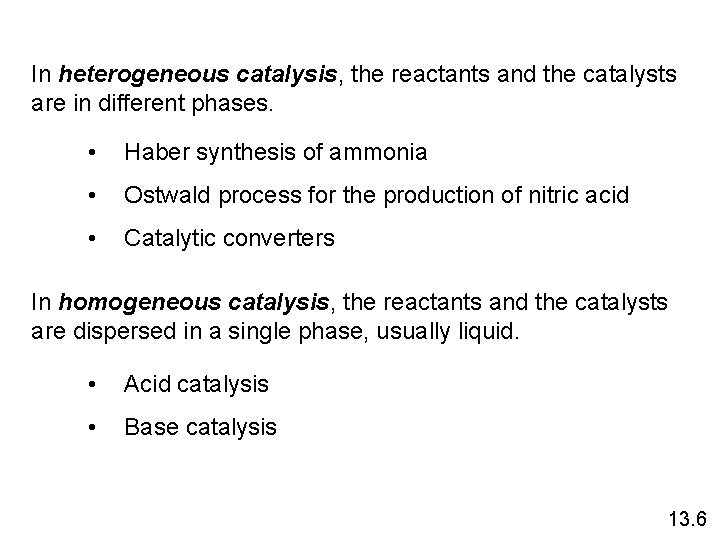
In heterogeneous catalysis, the reactants and the catalysts are in different phases. • Haber synthesis of ammonia • Ostwald process for the production of nitric acid • Catalytic converters In homogeneous catalysis, the reactants and the catalysts are dispersed in a single phase, usually liquid. • Acid catalysis • Base catalysis 13. 6
 A short poem that expresses an idea in a clever way is
A short poem that expresses an idea in a clever way is Chronology example paragraph
Chronology example paragraph When no air resistance acts on a projectile
When no air resistance acts on a projectile The menu expresses the concept and theme through
The menu expresses the concept and theme through Expresses action or being
Expresses action or being Expresses action
Expresses action A verb is a word that expresses
A verb is a word that expresses The designer expresses the ideas in terms related to the
The designer expresses the ideas in terms related to the Downward intonation
Downward intonation Which of these verbs expresses an action
Which of these verbs expresses an action Is a group of words that expresses a complete thought
Is a group of words that expresses a complete thought A verb expresses
A verb expresses Present simple expresses
Present simple expresses The designer expresses the ideas in terms related to the
The designer expresses the ideas in terms related to the The plot of oedipus deals mainly with
The plot of oedipus deals mainly with Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law V=k/p
V=k/p Constant of avogadro's law
Constant of avogadro's law Cap rate interest rate relationship
Cap rate interest rate relationship What is real interest rate and nominal interest rate
What is real interest rate and nominal interest rate