Kinetics Lesson 3 Rate Law Rate Law Equation


- Slides: 7

Kinetics Lesson #3 Rate Law

Rate Law Equation • A chemical rate law is an equation that connects the rate of a reaction with the concentration of its reactants at a given temperature and pressure. • If A + B → AB, then the rate is proportional to [A] x [B]. • Rate = k [A]m [B]n – Rate Law Equation • k is the rate constant (a specific value for each equation at a specific temperature). • [A] and [B] are the concentrations of the reactants • m and n are exponents determined experimentally that represent the importance or order of the reactants.

Rate Law Equation (continued) Note that k is not a true constant, as it changes with temperature, addition of catalyst, or change of catalyst. • The total order of reaction is the sum of the exponents in the rate law equation. • m and n are not at all related to molar coefficients from balanced equations.

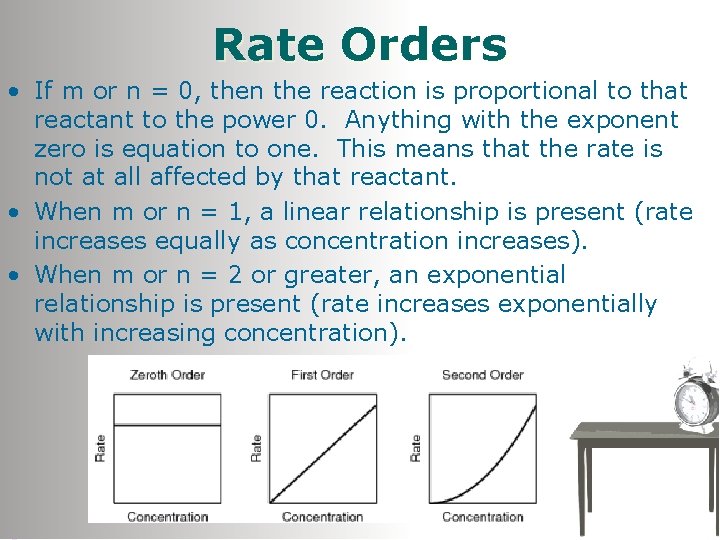
Rate Orders • If m or n = 0, then the reaction is proportional to that reactant to the power 0. Anything with the exponent zero is equation to one. This means that the rate is not at all affected by that reactant. • When m or n = 1, a linear relationship is present (rate increases equally as concentration increases). • When m or n = 2 or greater, an exponential relationship is present (rate increases exponentially with increasing concentration).

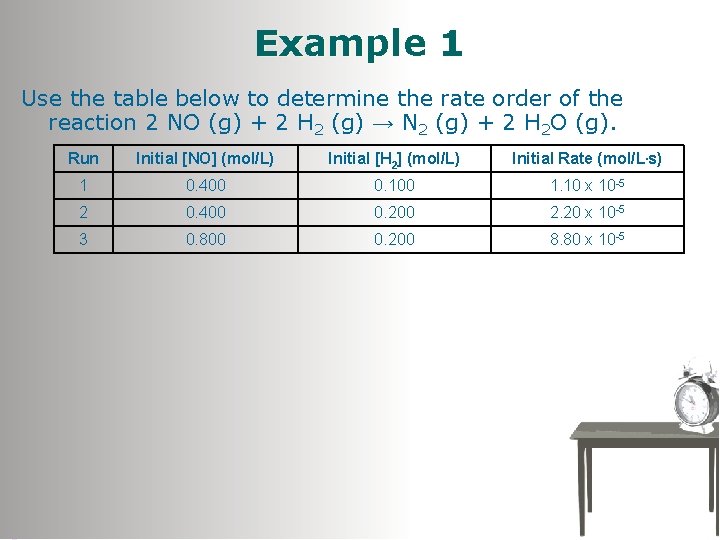
Example 1 Use the table below to determine the rate order of the reaction 2 NO (g) + 2 H 2 (g) → N 2 (g) + 2 H 2 O (g). Run Initial [NO] (mol/L) Initial [H 2] (mol/L) Initial Rate (mol/L. s) 1 0. 400 0. 100 1. 10 x 10 -5 2 0. 400 0. 200 2. 20 x 10 -5 3 0. 800 0. 200 8. 80 x 10 -5

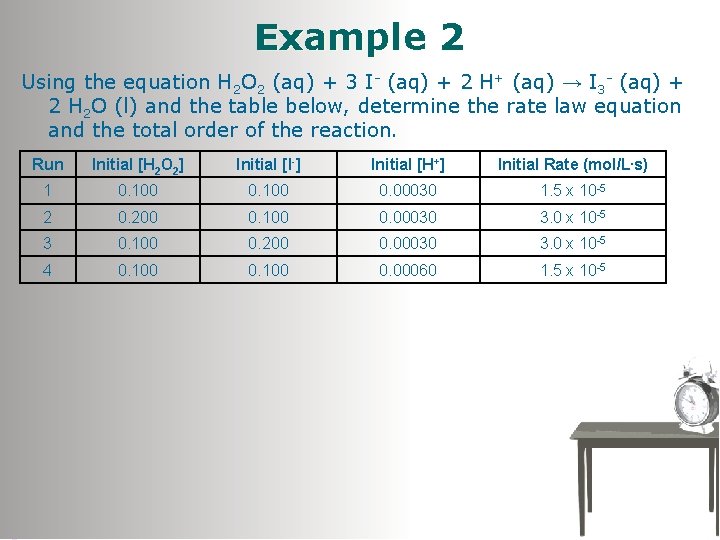
Example 2 Using the equation H 2 O 2 (aq) + 3 I- (aq) + 2 H+ (aq) → I 3 - (aq) + 2 H 2 O (l) and the table below, determine the rate law equation and the total order of the reaction. Run Initial [H 2 O 2] Initial [I-] Initial [H+] Initial Rate (mol/L. s) 1 0. 100 0. 00030 1. 5 x 10 -5 2 0. 200 0. 100 0. 00030 3. 0 x 10 -5 3 0. 100 0. 200 0. 00030 3. 0 x 10 -5 4 0. 100 0. 00060 1. 5 x 10 -5

Example 3 The initial rate of reaction for Br. O 3 - (aq) + 5 Br- (aq) + 6 H+ (aq) → 3 Br 2 (l) + 3 H 2 O (l) at 25°C is 3. 2 x 10 -3 mol/L when the initial concentration of Br. O 3 - is 0. 10 mol/L, Br- is 0. 10 mol/L and H+ is 0. 20 mol/L. The reaction is first order with respect to bromate and bromide, and second order with respect to the hydrogen ion. Determine the initial rate of reaction if the initial concentration of hydrogen ion is double with no change to the bromate or bromide ion concentration, and find the rate constant, k.