The national medication chart A summary of the



- Slides: 13

The national medication chart A summary of the 2012 changes NATIONAL MEDICATION SAFETY PROGRAMME

National medication chart • Review of original chart completed in March 2012 considered feedback from: – Change requests submitted by DHBs using the chart – Paediatric requirements consultation – Themes from the pilot of the long stay chart (16 days) • Revisions made to the chart retaining key safety features • Agreement to provide a suite of charts based on duration of stay in hospital – 8 day chart – for majority of patients – 16 day chart – for longer stay patients – 1 day chart – for emergency department and day stay patients

Review of the national medication chart • Key feedback messages: – Incorporate paediatric requirements into existing chart – no need for a separate chart – Investigate how we can view medicines succinctly – Incorporate venous thromboembolism prevention documentation into the chart – Increase the number of days to eight – Provide a longer stay and shorter stay chart – Include a prompt for prescribers to review prescriptions and re-prescribe – Make the dose box clearer for variable doses and decimal point – Make it scannable for electronic records

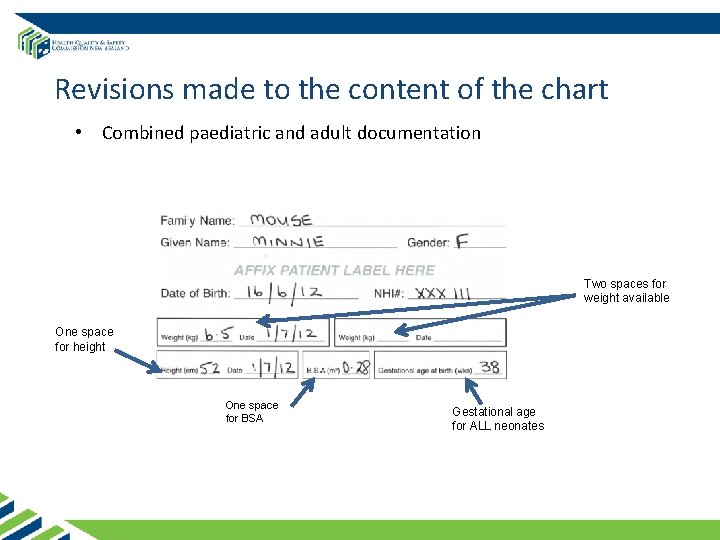
Revisions made to the content of the chart • Combined paediatric and adult documentation Two spaces for weight available One space for height One space for BSA Gestational age for ALL neonates

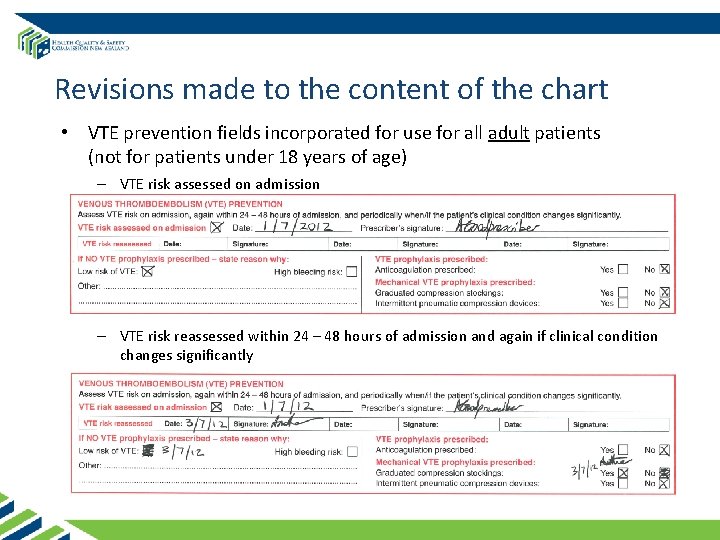
Revisions made to the content of the chart • VTE prevention fields incorporated for use for all adult patients (not for patients under 18 years of age) – VTE risk assessed on admission – VTE risk reassessed within 24 – 48 hours of admission and again if clinical condition changes significantly

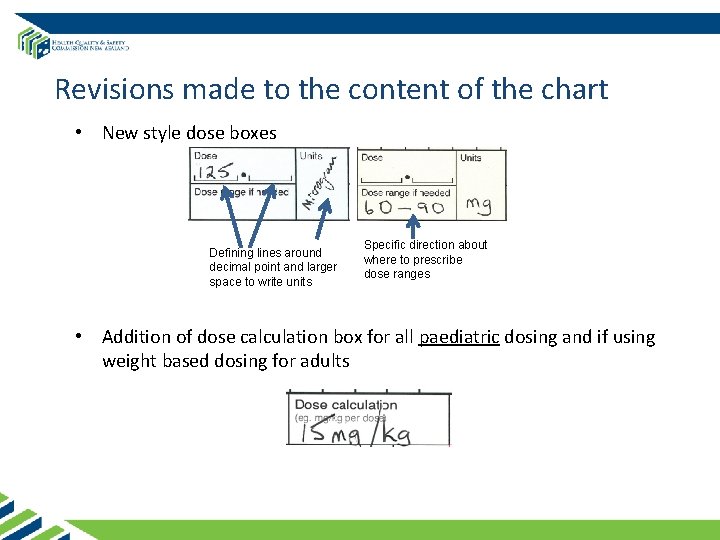
Revisions made to the content of the chart • New style dose boxes Defining lines around decimal point and larger space to write units Specific direction about where to prescribe dose ranges • Addition of dose calculation box for all paediatric dosing and if using weight based dosing for adults

Revisions made to the content of the chart • More medicines per page – Eight PRN and 23 regular medicine spaces • Increased duration – Chart now covers eight days – Additional 16 day chart for long stay patients • 16 day chart has – one PRN medicine page – three Regular medicine pages with fold out flaps

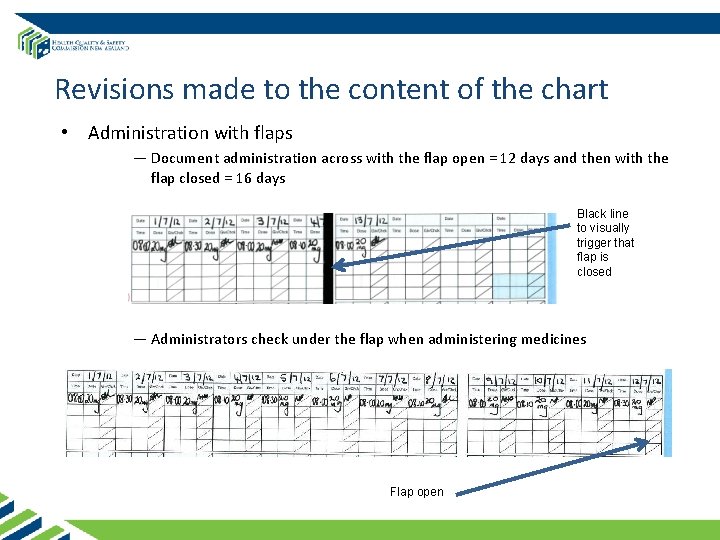
Revisions made to the content of the chart • Administration with flaps — Document administration across with the flap open = 12 days and then with the flap closed = 16 days Black line to visually trigger that flap is closed — Administrators check under the flap when administering medicines Flap open

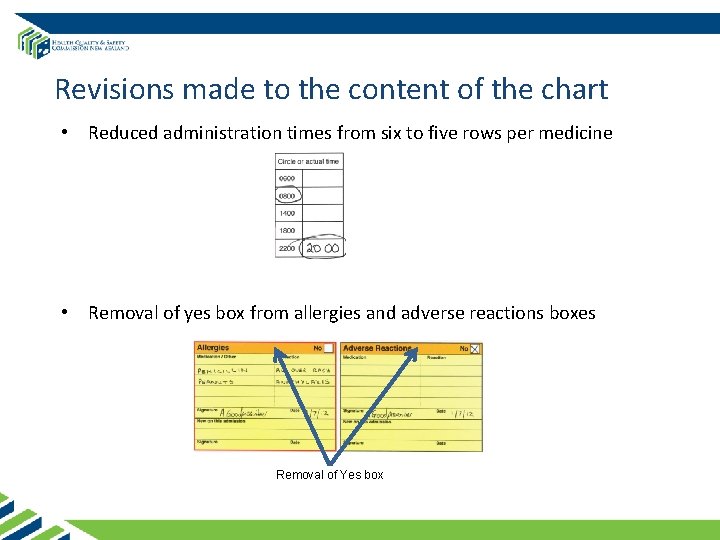
Revisions made to the content of the chart • Reduced administration times from six to five rows per medicine • Removal of yes box from allergies and adverse reactions boxes Removal of Yes box

Revisions made to the content of the chart • Prompt to re-prescribe on last administration day of medicines • Designs for scanning into electronic health record – Barcode on front page – Shaded grey area “do not write on this area” with scissors picture – Space to complete patient name and NHI on pages • six and nine of the 8 day chart • six and ten of the 16 day chart

Implementation status • 15 DHBs have implemented the chart into their hospitals. – A big thank you to the teams who have made this happen in their organisations – The 16 day and 1 day chart should enable these DHBs to expand to areas not currently using the national medication chart and fulfil the needs of long stay and short stay patients • Medication safety team is working with the remaining five DHBs to implement the national medication chart

Next steps • 8 day chart available for ordering from Wednesday 10 July 2012. – Same price structure as current national medication chart – Existing stocks of chart to be used first • 16 day chart available for ordering from Wednesday 25 July 2012 – Price structure more than current – DHBs will be sent a sample of the chart to assist with their decisions on what wards will use these • Posters outlining the changes will be available for DHBs to download • User guide for the national medication chart will be updated • Electronic Change Register will be made available to capture requests for the next review in two years time • 1 day chart to be developed and the aim is to have it available for ordering in November 2012

The Commission • Our website: www. hqsc. govt. nz • Register for our newsletter and updates • Contact us: info@hqsc. govt. nz