THE MOLE Measuring Matter What is a mole





- Slides: 8

THE MOLE Measuring Matter

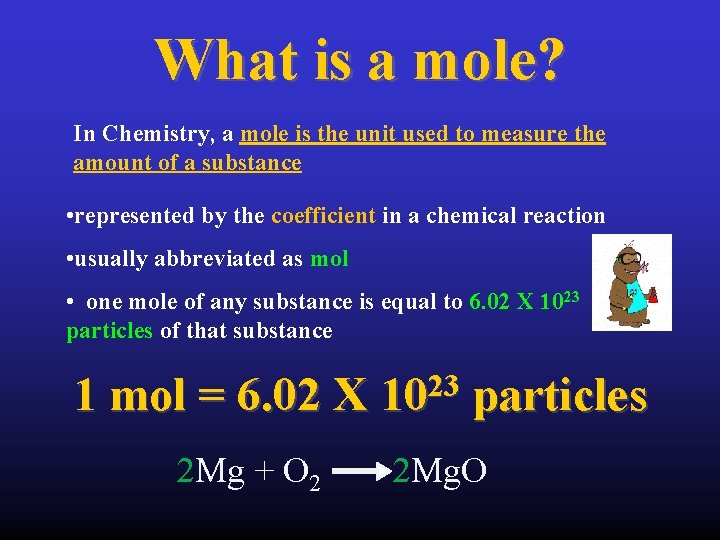
What is a mole? In Chemistry, a mole is the unit used to measure the amount of a substance • represented by the coefficient in a chemical reaction • usually abbreviated as mol • one mole of any substance is equal to 6. 02 X 1023 particles of that substance 1 mol = 6. 02 X 2 Mg + O 2 23 10 particles 2 Mg. O

What is 6. 02 X 1023 is called is equal to: 23 10 ? ? Avogadro’s number and 6. 02 X 10 X 10 X 10 X 10 X 10 X 10 Or 602, 000, 000, 000 Amadeo Avogadro was an Italian physicist who determined the volume of one mole of a gas in 1811

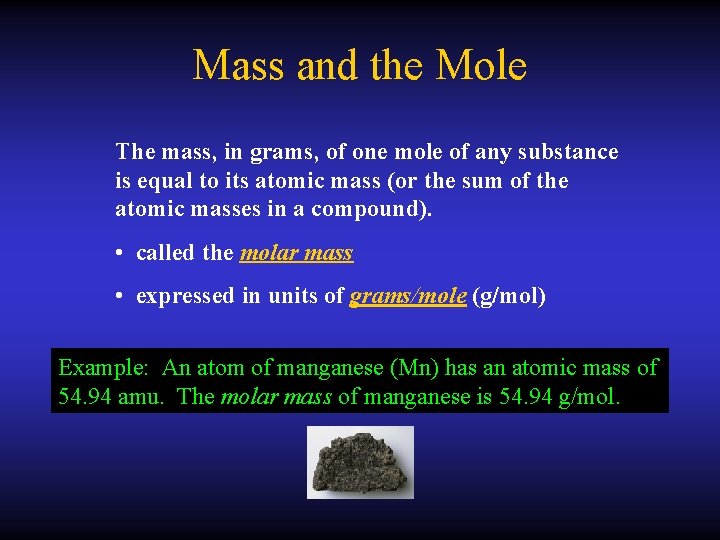
Mass and the Mole The mass, in grams, of one mole of any substance is equal to its atomic mass (or the sum of the atomic masses in a compound). • called the molar mass • expressed in units of grams/mole (g/mol) Example: An atom of manganese (Mn) has an atomic mass of 54. 94 amu. The molar mass of manganese is 54. 94 g/mol.

Using a conversion factor to convert from moles to mass How many grams of chromium (Cr) are there in 0. 0450 moles of that element? Chromium 1) From the Periodic Table, find the atomic mass of chromium. atomic mass Cr = 52. 00 amu 2) Determine the molar mass of chromium. molar mass Cr = 52. 00 g/mol 3) Create a conversion factor to do your calculations 0. 0450 mol Cr X 52. 00 g Cr 1 mol Cr = 2. 34 g Cr 52. 00 g Cr 1 mol Cr 24 Cr 52. 00

Converting from mass to moles In our previous example we converted 0. 0450 mol Cr to grams of Cr using the conversion factor: 52. 00 g Cr 1 mol Cr What conversion factor would we use if we were trying to convert a known mass (in grams) of Cr to moles? 1 mol Cr 52. 00 g Cr

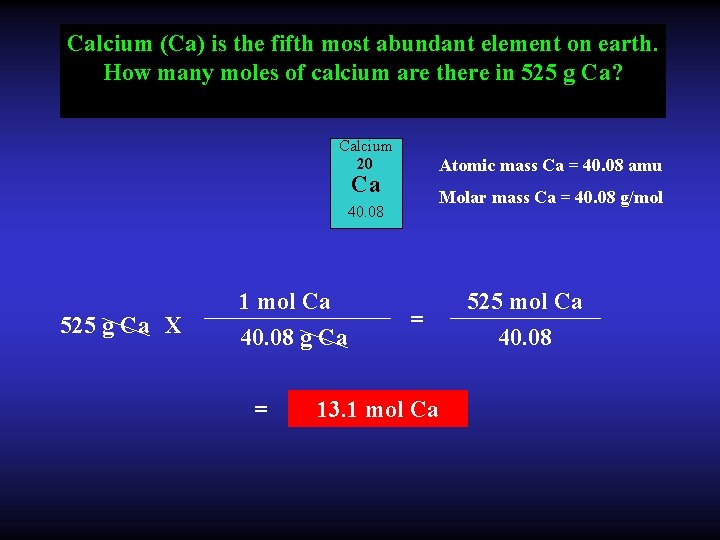
Calcium (Ca) is the fifth most abundant element on earth. How many moles of calcium are there in 525 g Ca? Calcium 20 Atomic mass Ca = 40. 08 amu Ca Molar mass Ca = 40. 08 g/mol 40. 08 525 g Ca X 1 mol Ca 40. 08 g Ca = = 13. 1 mol Ca 525 mol Ca 40. 08

Practice 1) How many moles of Silver (Ag) are there in 25. 5 g Ag? 0. 236 mol Ag 2) Determine the mass, in grams, of 3. 45 mol Cobalt (Co) 203 g Co BONUS for the REAL thinker: How many atoms of gold (Au) would there be in 25. 0 g of the metal? 7. 65 X 1022 atoms Au How did the REAL thinkers get this?