The Mole Measurement of Matter Measuring Matter How











- Slides: 15

The Mole Measurement of Matter

Measuring Matter • How do we measure matter? • Count, Mass, or Volume • • • Counting: 1 dozen apples = 12 apples Mass: 1 dz. Apples = 2. 0 kg apples Volume: 1 dz. Apples = 0. 20 bushels apples

Unit Conversions • Relating to a dozen allows you to convert between the units • Unit Conversion Factors

What is the Mole? • A counting number (like a dozen) • Avogadro’s number (NA) • 1 mol = 6. 02 1023 items

Size of Moles • 1 mole of pennies would cover the Earth 1/4 mile deep! n 1 mole of hockey pucks would equal the mass of the moon! n 1 mole of basketballs would fill a bag the size of the earth!

Molar Mass • Mass of 1 mole of an element or compound. • Atomic mass tells the. . . • atomic mass units per atom (amu) • grams per mole (g/mol)

Molar Mass Examples • carbon 12. 01 g/mol • aluminum 26. 98 g/mol • zinc 65. 39 g/mol

Molar Mass Examples • water · H 2 O · 2(1. 01) + 16. 00 = 18. 02 g/mol • sodium chloride · Na. Cl · 22. 99 + 35. 45 = 58. 44 g/mol

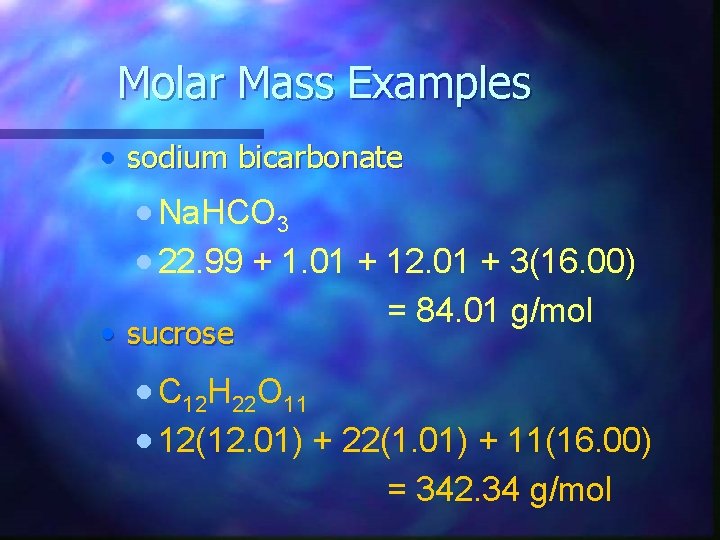
Molar Mass Examples • sodium bicarbonate · Na. HCO 3 · 22. 99 + 1. 01 + 12. 01 + 3(16. 00) • sucrose = 84. 01 g/mol · C 12 H 22 O 11 · 12(12. 01) + 22(1. 01) + 11(16. 00) = 342. 34 g/mol

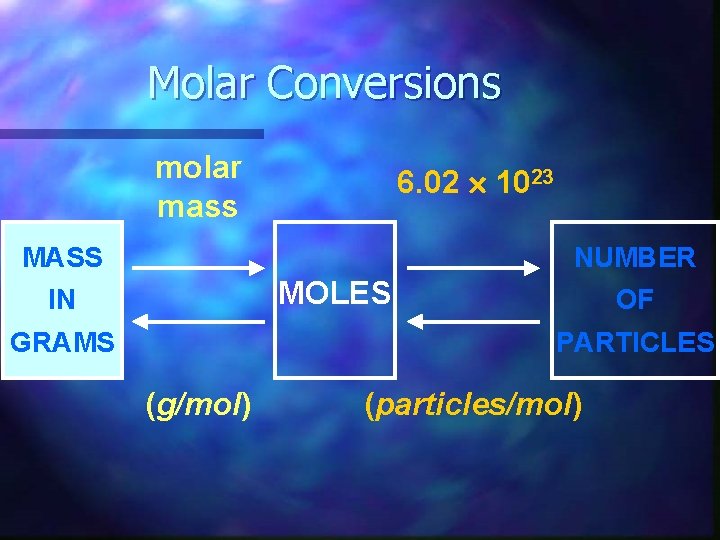
Molar Conversions molar mass 6. 02 1023 MASS NUMBER MOLES IN GRAMS OF PARTICLES (g/mol) (particles/mol)

Molar Conversion Examples • How many moles of carbon are in 26 g of carbon? 26 g C 1 mol C 12. 01 g C = 2. 2 mol C

Molar Conversion Examples • How many molecules are in 2. 50 moles of C 12 H 22 O 11? 6. 02 1023 2. 50 molecules 1 mol = 1. 51 1024 molecules C 12 H 22 O 11

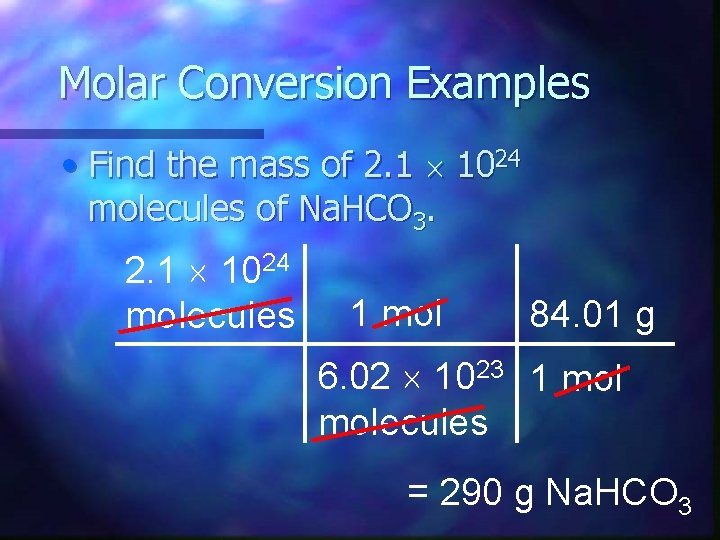
Molar Conversion Examples • Find the mass of 2. 1 1024 molecules of Na. HCO 3. 2. 1 1024 molecules 1 mol 84. 01 g 6. 02 1023 1 molecules = 290 g Na. HCO 3

The Mole and Avogadro’s Number • Mole: unit for defining a group of atoms • Avogadro’s number = 6. 022 x 1023 • 1 mole of atoms = 6. 022 x 1023 atoms • Molar mass - The mass (g) of 1 mole of atoms • What is the molar mass of carbon? 12. 01 g/mol C • One mole of any element contains the same number of atoms, 6. 022 x 1023, Avogadro’s number

Practice Calculations 1. Calculate the number of atoms in 1. 7 moles of boron. 2. Find the mass in grams of 2. 5 mol Na (sodium). 3. Calculate the number of atoms in 5. 0 g aluminum. 4. Calculate the mass of 5, 000 atoms of Au (gold)
 The mole measuring matter
The mole measuring matter The mole measuring matter
The mole measuring matter The mole a measurement of matter
The mole a measurement of matter 10.1 the mole: a measurement of matter
10.1 the mole: a measurement of matter 10.1 the mole a measurement of matter
10.1 the mole a measurement of matter Mole-mass-volume relationships
Mole-mass-volume relationships Phosphorus and oxygen equation
Phosphorus and oxygen equation Grams to gram
Grams to gram Stoichiometry mole-mole
Stoichiometry mole-mole Mole mole factor
Mole mole factor Molar mass to grams
Molar mass to grams Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole unit of measurement
Mole unit of measurement Mole unit of measurement
Mole unit of measurement Mole measurement examples
Mole measurement examples Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key