The Impact of Preoperative Renal Dysfunction on the




- Slides: 12

The Impact of Preoperative Renal Dysfunction on the Outcomes of Patients Undergoing Transcatheter Aortic Valve Replacement Andres M. Pineda MD, J. Kevin Harrison MD, Neil S. Kleiman MD, Michael J. Reardon, MD, John V. Conte, MD, Daniel P. O’Hair, MD, Stanley Chetcuti, MD, Steven J. Yakubov, MD, Jeffrey J. Popma, MD, Nirat Beohar MD; For the Core. Valve US Clinical Investigators

Background • The impact of renal dysfunction severity using baseline glomerular filtration rate (GFR) on outcomes after TAVR remains unknown. • Similarly, the impact of preoperative renal dysfunction on the clinical outcomes after TAVR vs. SAVR is unclear.

Methods • To analyze outcomes of TAVR patients, all patients undergoing TAVR in the Core. Valve US Pivotal Extreme Risk Trial, High Risk Trial, and Continued Access Studies were pooled, and stratified by baseline GFR as none/mild (GFR > 60 m. L/min/1. 73 m 2), moderate (GFR 30 -60), or severe (GFR < 30) preoperative renal dysfunction. The Cr. Cl/GFR for all patients was calculated by the MDRD equation and the baseline SCr. • To compare TAVR vs. SAVR, patients enrolled in the Core. Valve US Pivotal High Risk Trial were stratified by pre-operative renal dysfunction as described above. • The primary endpoint was the incidence of major adverse cardiovascular and renal events (MACRE); defined as all-cause mortality, myocardial infarction, stroke/TIA, and need for new renal replacement therapy.

Statistical Methods • Categorical variables were compared using the Fisher exact test or the chi-square test. Continuous variables are presented as mean ± standard deviation, compared using a t test. Kaplan-Meier estimates were used to construct survival and MACRE curves based on all available follow-up data for the time-to-event analysis. Differences in event rates were evaluated using the log-rank test. All testing used a 2 -sided α level of 0. 05. • For the predictors of mortality analysis, all TAVR patients with GFR ≤ 60 m. L/min/1. 73 m 2 were included; variables were selected based on clinical relevance. Cox proportional hazards survival models were used for 30 -day and 1 -year mortality. Multivariable predictors were identified from univariable predictors with p value ≤ 0. 05. A stepwise procedure was performed to determine the final model. The significance level thresholds for entry and exit of independent variables were set at 0. 10.

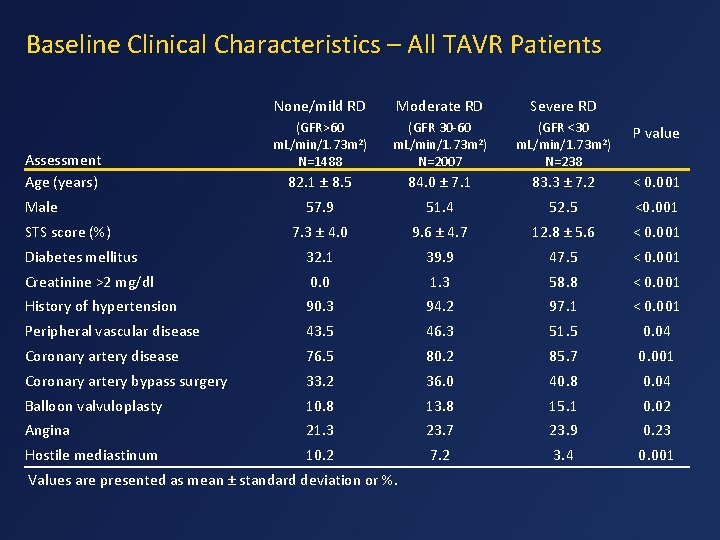
Baseline Clinical Characteristics – All TAVR Patients None/mild RD Moderate RD Severe RD (GFR>60 m. L/min/1. 73 m 2) N=1488 (GFR 30 -60 m. L/min/1. 73 m 2) N=2007 (GFR <30 m. L/min/1. 73 m 2) N=238 P value 82. 1 ± 8. 5 84. 0 ± 7. 1 83. 3 ± 7. 2 < 0. 001 57. 9 51. 4 52. 5 <0. 001 7. 3 ± 4. 0 9. 6 ± 4. 7 12. 8 ± 5. 6 < 0. 001 Diabetes mellitus 32. 1 39. 9 47. 5 < 0. 001 Creatinine >2 mg/dl 0. 0 1. 3 58. 8 < 0. 001 History of hypertension 90. 3 94. 2 97. 1 < 0. 001 Peripheral vascular disease 43. 5 46. 3 51. 5 0. 04 Coronary artery disease 76. 5 80. 2 85. 7 0. 001 Coronary artery bypass surgery 33. 2 36. 0 40. 8 0. 04 Balloon valvuloplasty 10. 8 13. 8 15. 1 0. 02 Angina 21. 3 23. 7 23. 9 0. 23 Hostile mediastinum 10. 2 7. 2 3. 4 0. 001 Assessment Age (years) Male STS score (%) Values are presented as mean ± standard deviation or %.

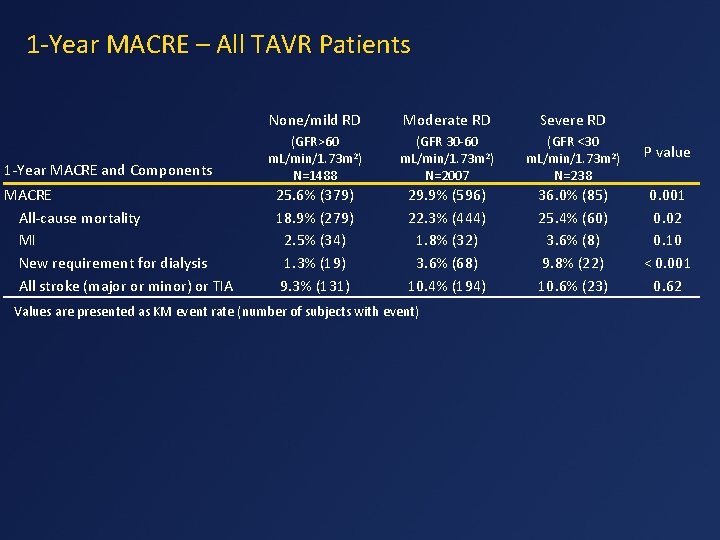
1 -Year MACRE – All TAVR Patients 1 -Year MACRE and Components MACRE All-cause mortality MI New requirement for dialysis All stroke (major or minor) or TIA None/mild RD Moderate RD Severe RD (GFR>60 m. L/min/1. 73 m 2) N=1488 (GFR 30 -60 m. L/min/1. 73 m 2) N=2007 (GFR <30 m. L/min/1. 73 m 2) N=238 P value 25. 6% (379) 18. 9% (279) 2. 5% (34) 1. 3% (19) 9. 3% (131) 29. 9% (596) 22. 3% (444) 1. 8% (32) 3. 6% (68) 10. 4% (194) 36. 0% (85) 25. 4% (60) 3. 6% (8) 9. 8% (22) 10. 6% (23) 0. 001 0. 02 0. 10 < 0. 001 0. 62 Values are presented as KM event rate (number of subjects with event)

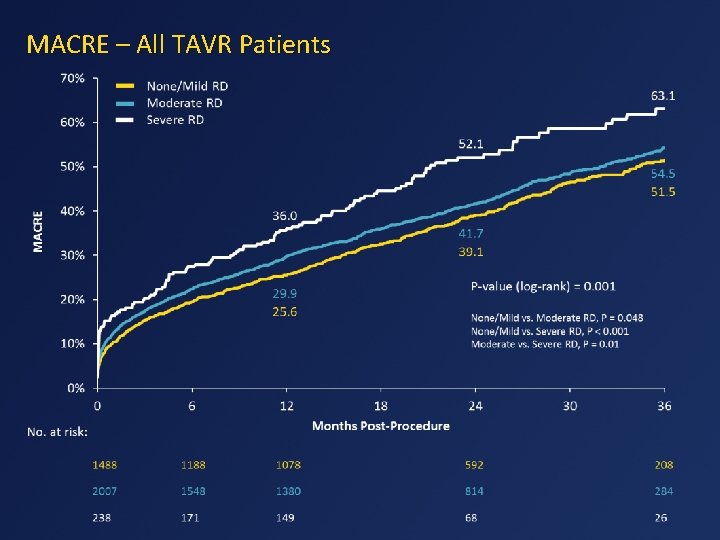
MACRE – All TAVR Patients

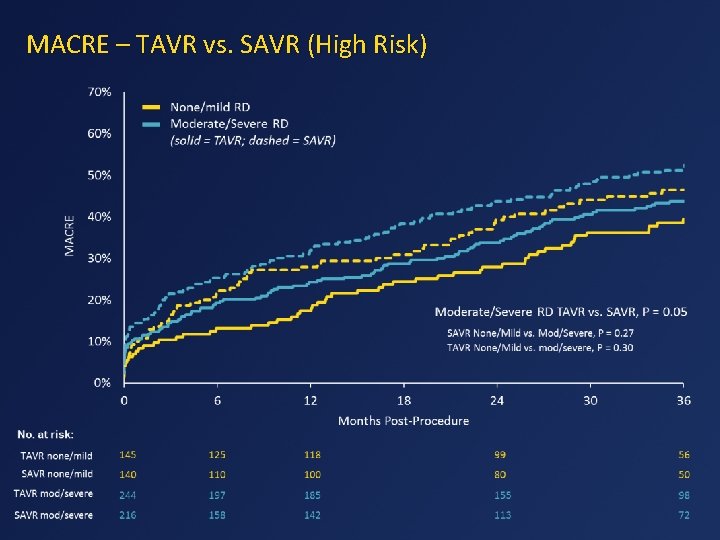
MACRE – TAVR vs. SAVR (High Risk)

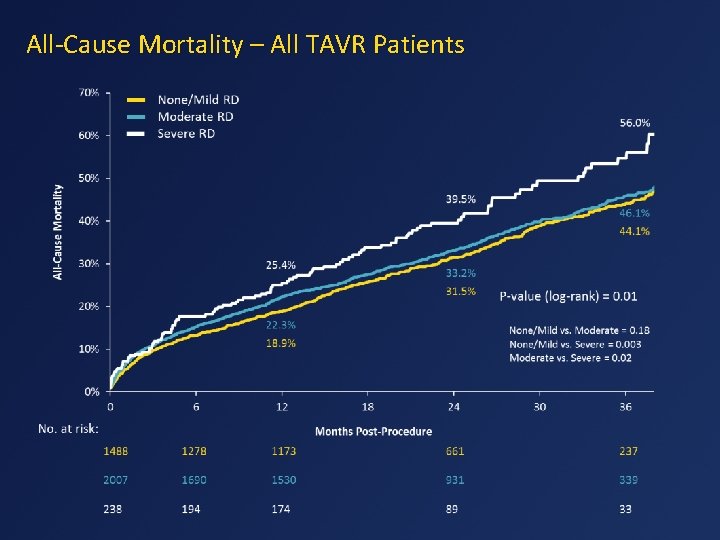
All-Cause Mortality – All TAVR Patients

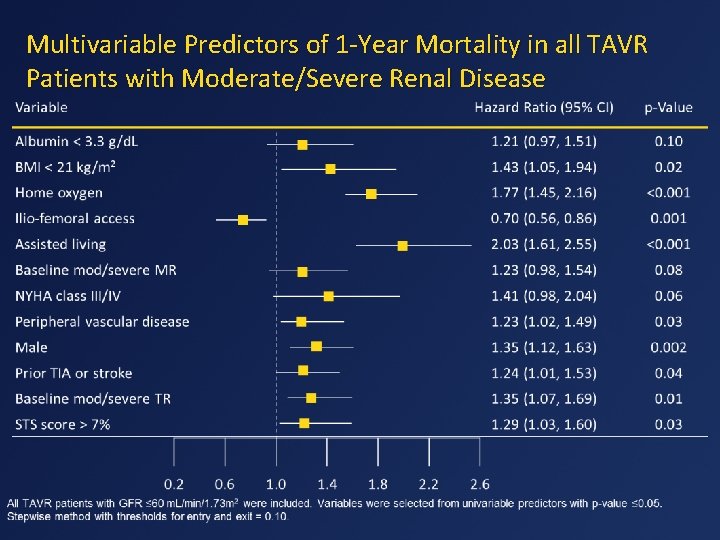
Multivariable Predictors of 1 -Year Mortality in all TAVR Patients with Moderate/Severe Renal Disease

Conclusions • Worse baseline renal function increases mortality and the MACRE composite endpoint in TAVR patients. • TAVR has lower rates of major adverse events compared with SAVR in those with none to moderate preoperative renal dysfunction. • In patients undergoing TAVR with GFR ≤ 60, several independent predictors of 1 year mortality were identified, including male sex, NYHA III-IV, peripheral vascular disease, prior stroke or TIA, STS >7% and non-iliofemoral access, amongst others.

Disclosures • The study was funded by Medtronic (Minneapolis, MN). • Medtronic personnel performed all statistical analysis, verified the accuracy of the data presented, and assisted in the graphical display of the data. • Dr. Pineda and Dr. Beohar have no disclosures. Dr. Harrison has received institutional grants from Medtronic, Boston Scientific, Direct Flow Medical, St. Jude Medical, and Edwards Lifesciences; and serves on a Medical Advisory Board for Direct Flow Medical and on the Data Safety Monitoring Board for Cardi. AQ. Dr. Kleiman and Dr. Reardon have received fees from Medtronic for providing educational services. Dr. Conte serves on a surgical advisory board for Medtronic and Sorin. Dr. O’Hair reports receiving grant support from Medtronic and Edwards Lifesciences. Dr. Chetcuti has received grant support from Edwards Lifesciences, Boston Scientific, and Medtronic, and has received proctoring fees from Medtronic. Dr. Yakubov, has received grant support and served on advisory boards for Medtronic and Boston Scientific, and has received grant support from Direct Flow Medical. Dr. Popma has received grants from Medtronic, Boston Scientific, and Direct Flow Medical.
 Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Distinguish between renal corpuscle and renal tubule
Distinguish between renal corpuscle and renal tubule Ivrt echo
Ivrt echo Reactive airways dysfunction syndrome
Reactive airways dysfunction syndrome Impaired mentation
Impaired mentation Loulwah mukharesh
Loulwah mukharesh Arousal disorder symptoms
Arousal disorder symptoms Somatic dysfunction in osteopathic family medicine
Somatic dysfunction in osteopathic family medicine Rib somatic dysfunction
Rib somatic dysfunction Breathing pattern dysfunction
Breathing pattern dysfunction Antigliadin antibodies
Antigliadin antibodies Rib somatic dysfunction
Rib somatic dysfunction























