The Chemistry of Life What are living creatures



- Slides: 19

The Chemistry of Life What are living creatures made of? Why do we have to eat?

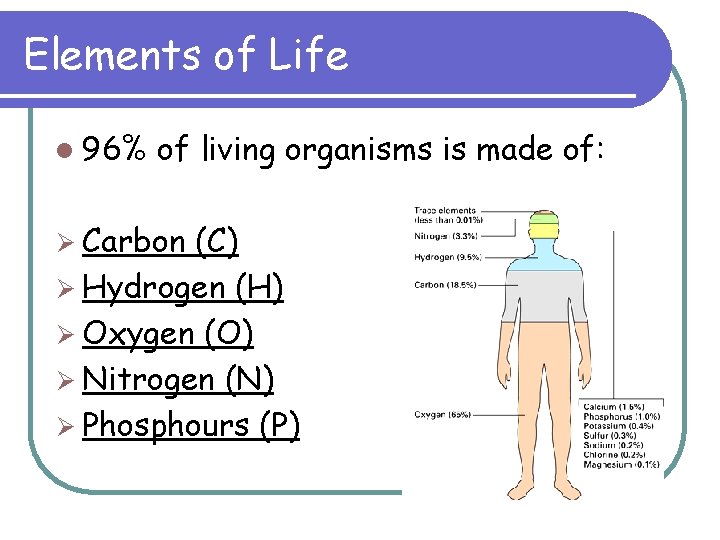
Elements of Life l 96% of living organisms is made of: Ø Carbon (C) Ø Hydrogen (H) Ø Oxygen (O) Ø Nitrogen (N) Ø Phosphours (P)

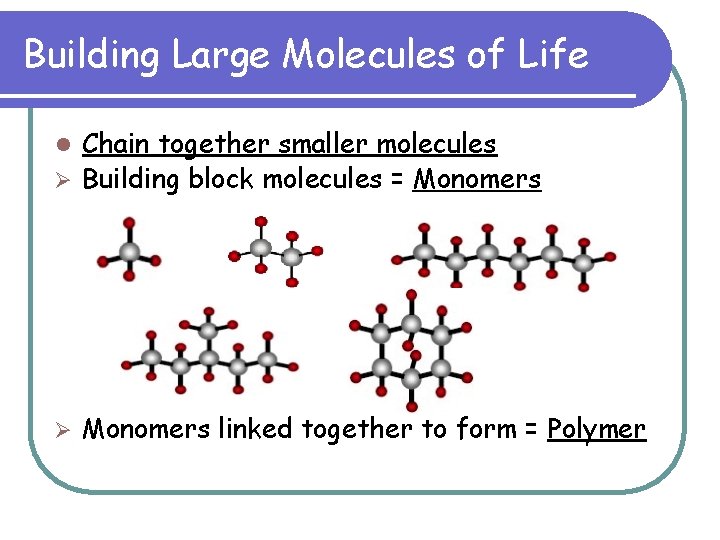
Building Large Molecules of Life Chain together smaller molecules Ø Building block molecules = Monomers l Ø Monomers linked together to form = Polymer

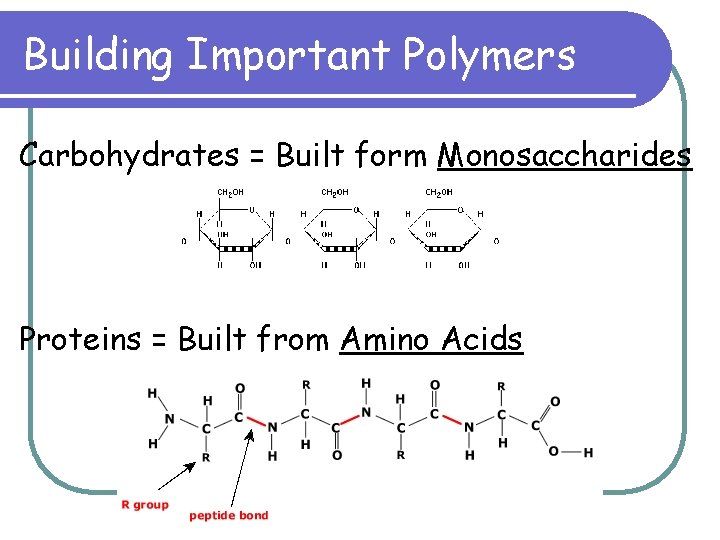
Building Important Polymers Carbohydrates = Built form Monosaccharides Proteins = Built from Amino Acids

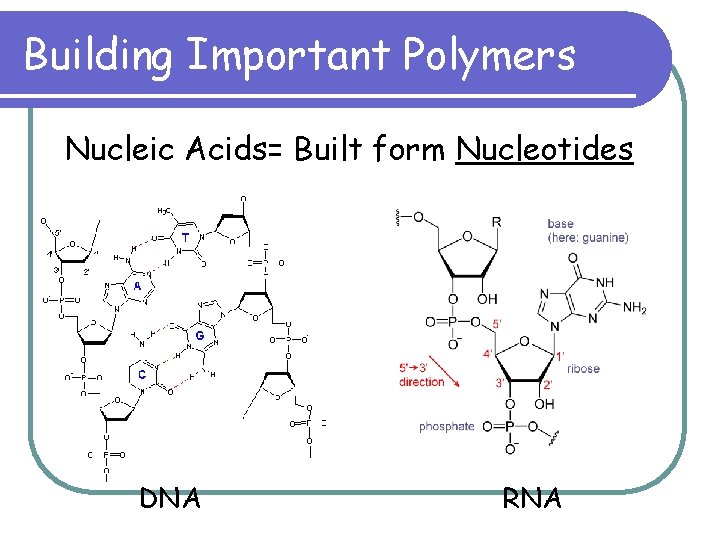
Building Important Polymers Nucleic Acids= Built form Nucleotides DNA RNA

Carbohydrates

General characteristics of Carbohydrates l Compounds composed of element C, H, and O l The ratio of carbon atoms to hydrogen atoms to oxygen atoms in every single carbohydrate is 1: 2: 1, the simplest carbohydrate has the molecular formula of CH 2 O l All sugars have the ending -ose e. g. glucose, galactose l carbohydrates are the most abundant compounds found in nature (E. g. cellulose: 100 billion tons annually)

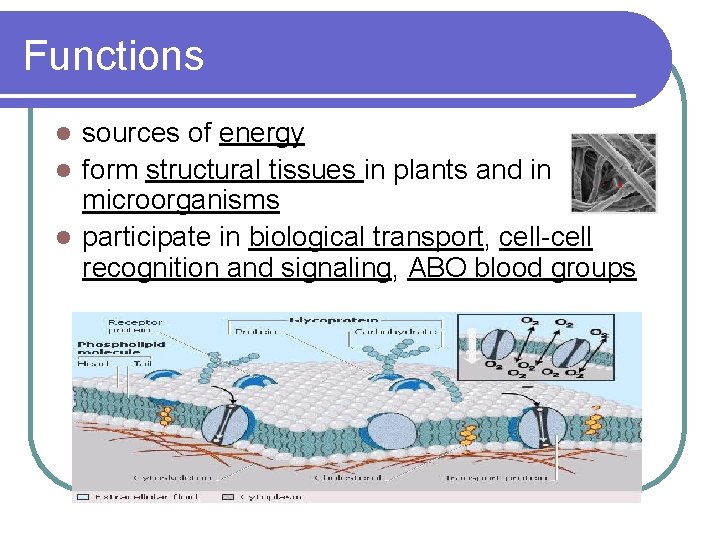
Functions sources of energy l form structural tissues in plants and in microorganisms l participate in biological transport, cell-cell recognition and signaling, ABO blood groups l

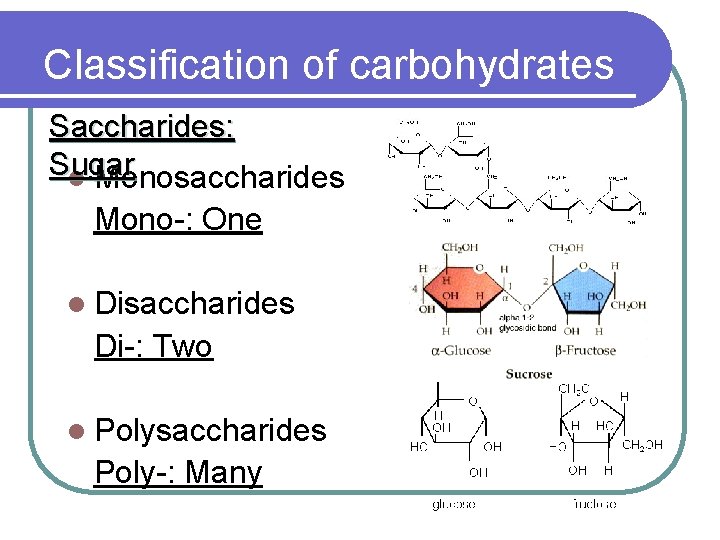
Classification of carbohydrates Saccharides: Sugar l Monosaccharides Mono-: One l Disaccharides Di-: Two l Polysaccharides Poly-: Many

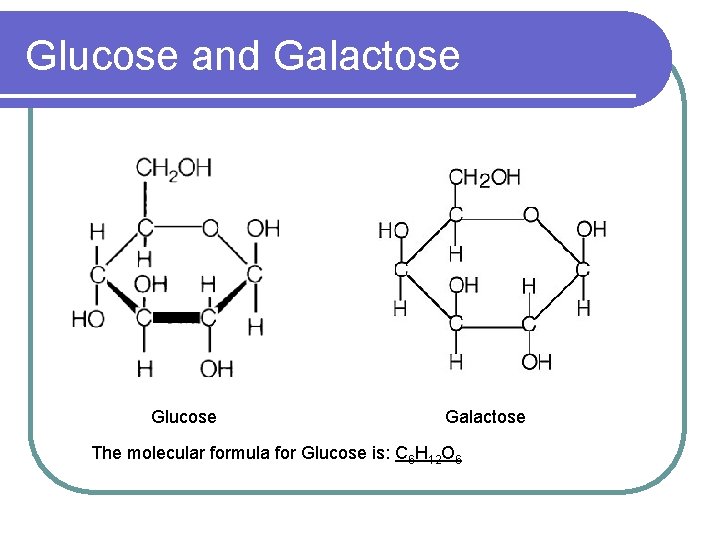
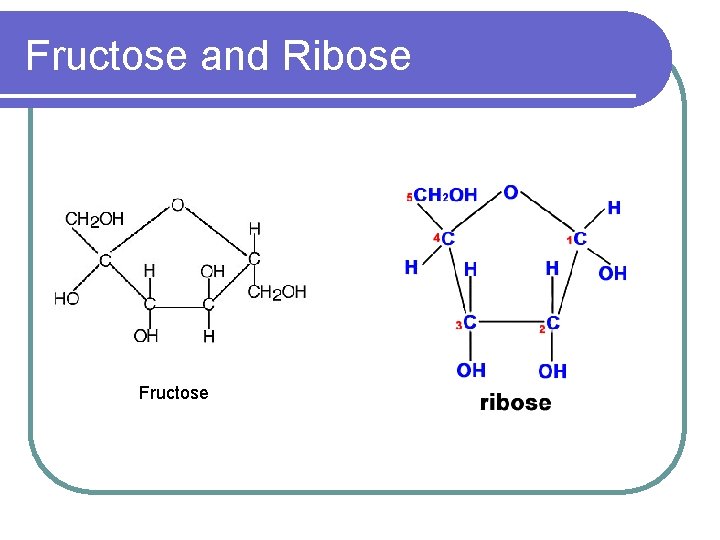
Monosaccharides l Also known as simple sugars l Basic unit of any carbohydrates l Example: Glucose, galactose, fructose, ribose

Glucose and Galactose Glucose Galactose The molecular formula for Glucose is: C 6 H 12 O 6

Fructose and Ribose Fructose

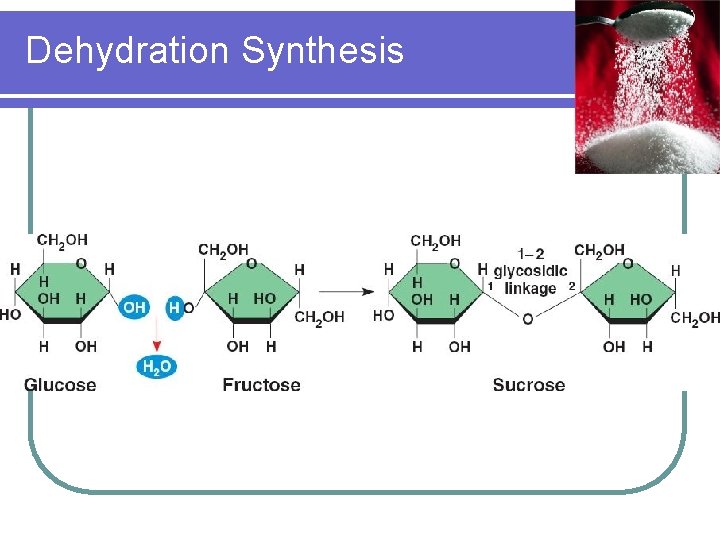
Disaccharides l Disaccharides are the products when two monosaccharides are chemically linked together with the loss of one water molecule l This chemical reaction is called dehydration synthesis l One example of disaccharides is sucrose, also known as table sugar

Dehydration Synthesis

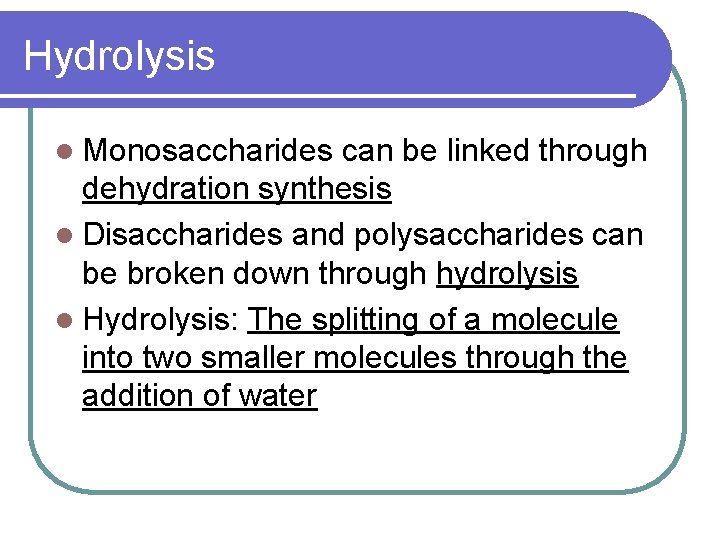
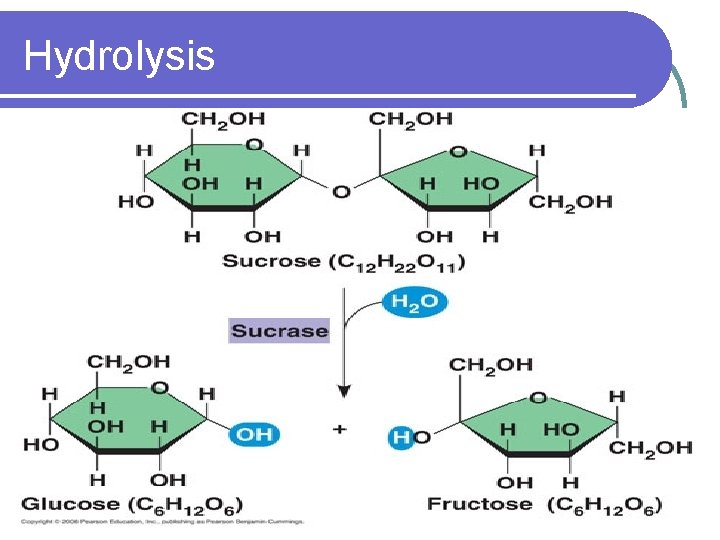
Hydrolysis l Monosaccharides can be linked through dehydration synthesis l Disaccharides and polysaccharides can be broken down through hydrolysis l Hydrolysis: The splitting of a molecule into two smaller molecules through the addition of water

Hydrolysis

Compare and Contrast l Dehydration Synthesis l Hydrolysis

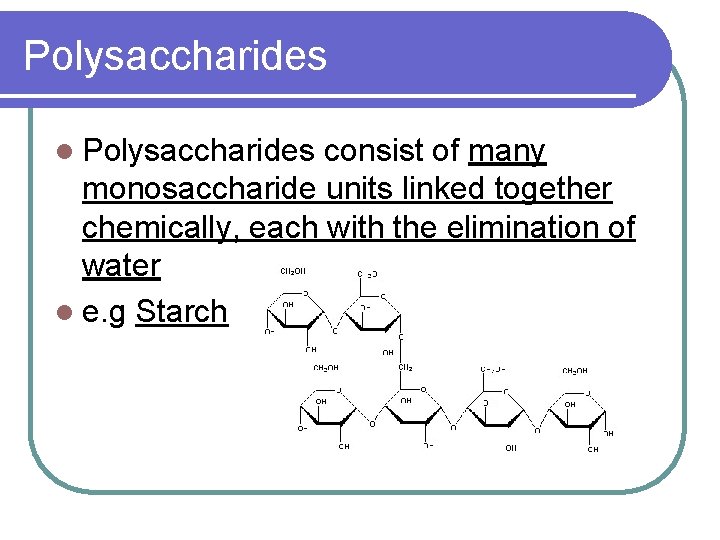
Polysaccharides l Polysaccharides consist of many monosaccharide units linked together chemically, each with the elimination of water l e. g Starch

Relative Sweetness l Fructose > Sucrose > Lactose l How do we know?
 Antigentest åre
Antigentest åre Four living creatures covered with eyes
Four living creatures covered with eyes Small living creatures tom 20
Small living creatures tom 20 Small living creatures tom 23
Small living creatures tom 23 Organization of living things
Organization of living things Whats an energy pyramid
Whats an energy pyramid Tomato living or nonliving
Tomato living or nonliving Living non living dead
Living non living dead Smallest living unit of life
Smallest living unit of life Living by chemistry unit 2 smells answers
Living by chemistry unit 2 smells answers Living by chemistry answer key
Living by chemistry answer key Living by chemistry solutions
Living by chemistry solutions What is catalystfive
What is catalystfive Lesson 81 drop in molecular views
Lesson 81 drop in molecular views Living by chemistry
Living by chemistry Living by chemistry
Living by chemistry Lesson 20 getting connected ionic compounds
Lesson 20 getting connected ionic compounds Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Rock pool creatures
Rock pool creatures