Test Review Physical properties of Matter Definitions Ductility




- Slides: 9

Test Review Physical properties of Matter

Definitions Ductility- Can be pulled into a wire (property of metals) Luster- Reflects light, shiny (property of metals) Malleability- the ability to be rolled or pounded into thin sheets, like aluminum foil (property of metals) Conductivity- the ability to transfer thermal energy (heat) or electricity(property of metals) Streak- the mineral in powder form, use a streak plate (property of minerals) Hardiness- the ability for a mineral to be scratched, 1 -10 and 10 is the hardest

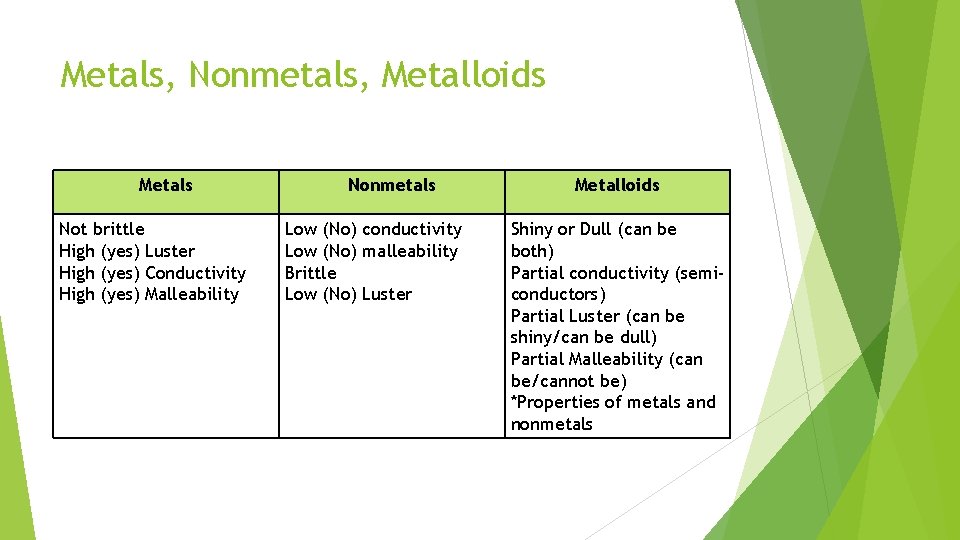
Metals, Nonmetals, Metalloids Metals Not brittle High (yes) Luster High (yes) Conductivity High (yes) Malleability Nonmetals Low (No) conductivity Low (No) malleability Brittle Low (No) Luster Metalloids Shiny or Dull (can be both) Partial conductivity (semiconductors) Partial Luster (can be shiny/can be dull) Partial Malleability (can be/cannot be) *Properties of metals and nonmetals

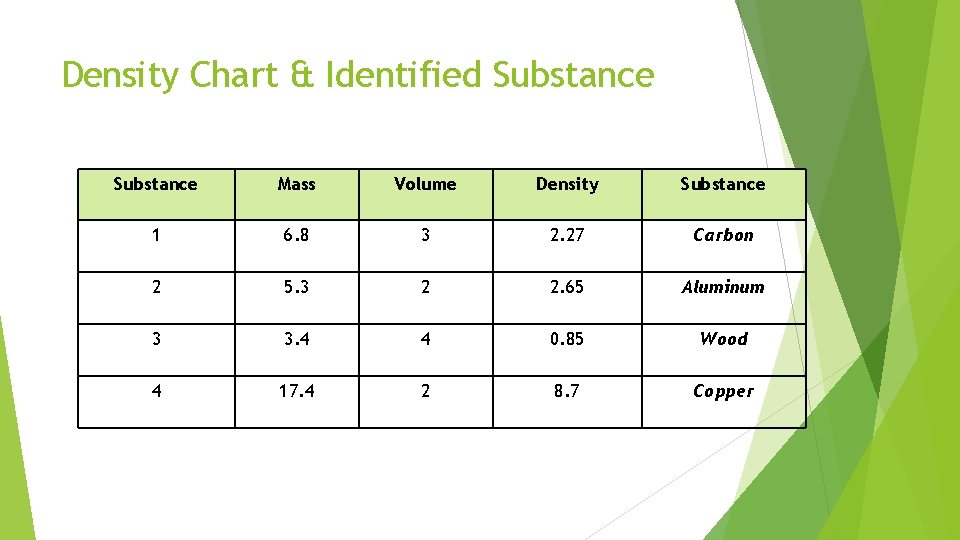
Density Chart & Identified Substance Mass Volume Density Substance 1 6. 8 3 2. 27 Carbon 2 5. 3 2 2. 65 Aluminum 3 3. 4 4 0. 85 Wood 4 17. 4 2 8. 7 Copper

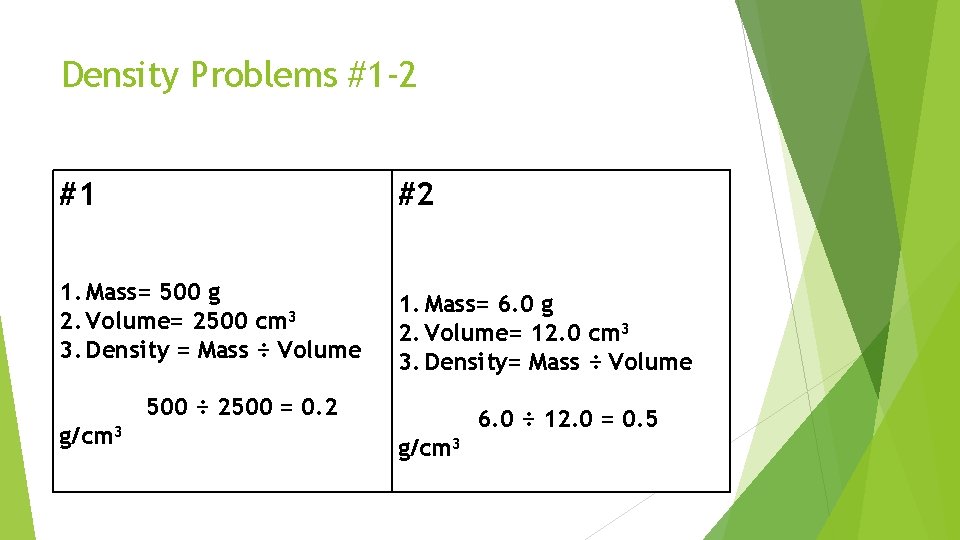
Density Problems #1 -2 #1 #2 1. Mass= 500 g 2. Volume= 2500 cm 3 3. Density = Mass ÷ Volume 1. Mass= 6. 0 g 2. Volume= 12. 0 cm 3 3. Density= Mass ÷ Volume 500 ÷ 2500 = 0. 2 g/cm 3 6. 0 ÷ 12. 0 = 0. 5 g/cm 3

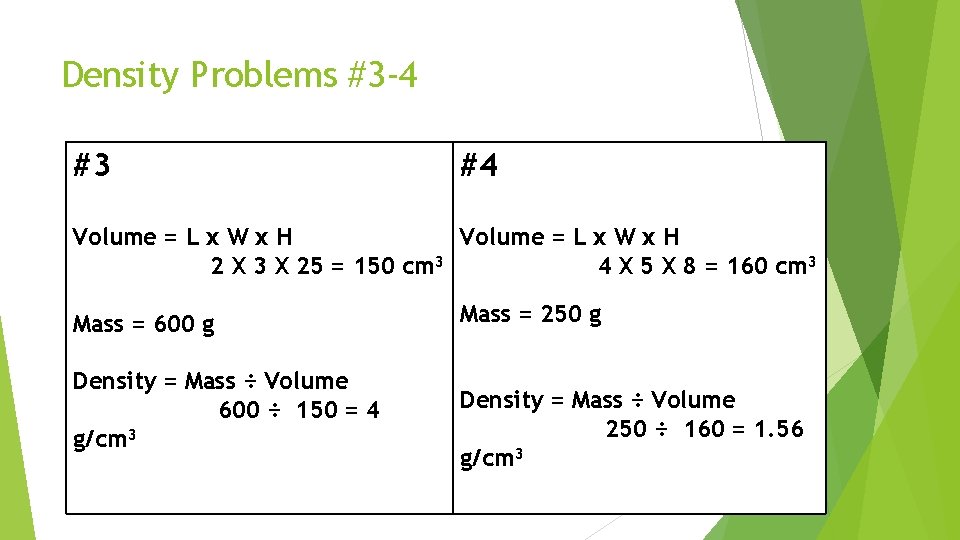
Density Problems #3 -4 #3 #4 Volume = L x W x H 2 X 3 X 25 = 150 cm 3 4 X 5 X 8 = 160 cm 3 Mass = 600 g Density = Mass ÷ Volume 600 ÷ 150 = 4 g/cm 3 Mass = 250 g Density = Mass ÷ Volume 250 ÷ 160 = 1. 56 g/cm 3

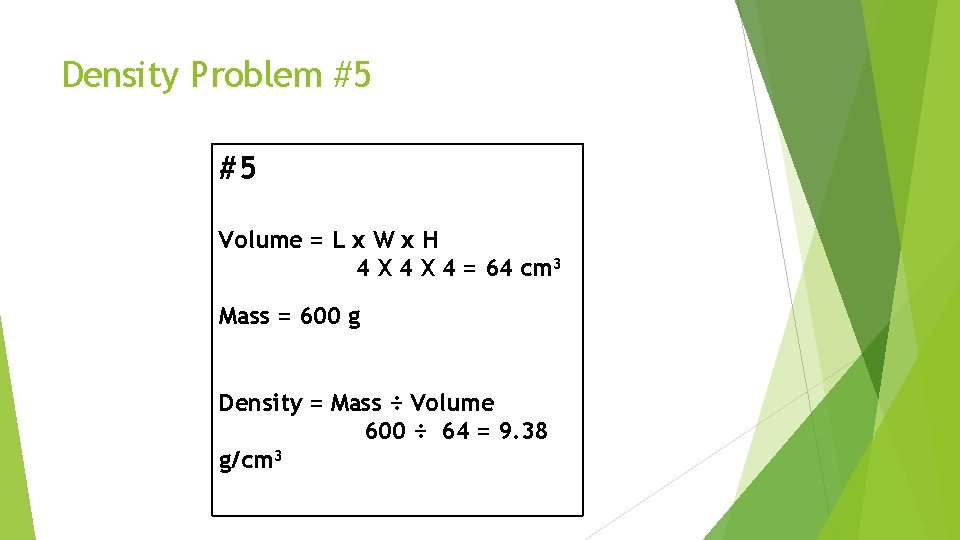
Density Problem #5 #5 Volume = L x W x H 4 X 4 = 64 cm 3 Mass = 600 g Density = Mass ÷ Volume 600 ÷ 64 = 9. 38 g/cm 3

Minerals Quartz= 7, it is harder than Feldspar=6 Feldspar can scratch glass but not the streak plate (feldspar is between the glass and sand) Flourite is harder than calcite but will not scratch the glass (It is between calcite and apatite/glass) Hematite is a 6 (It is between the glass 5. 5 and steel 6. 5)

Physical properties for Minerals A. Color B. Luster C. Streak D. Hardness E. Cleavage F. Fracture