Technologies for Radon Radionuclide Removal Tom Sorg U
























- Slides: 28

Technologies for Radon & Radionuclide Removal Tom Sorg U. S. Environmental Protection Agency

Radionuclides Radon Rn Radium Ra Uranium U

Radon - 222 Radioactive Element in the Uranium 238 decay series Decay product of Ra 226 Alpha emitter Half life of 3. 8 days

Radon - 222 Rn 222 3. 8 days Po 218 3 min Pb 214 27 min Bi 214 20 min Po 214 1. 6 x 10 -6 sec Pb 210 20 years

Radon - 222 Gas Naturally occurring ground water contaminant Proposed MCL - 300 p. Ci/L MMM Program - 4000 p. Ci/L (AMCL)

Radon Removal Technology Aeration (BAT) 70 - 99 % GAC 80 - 99 %

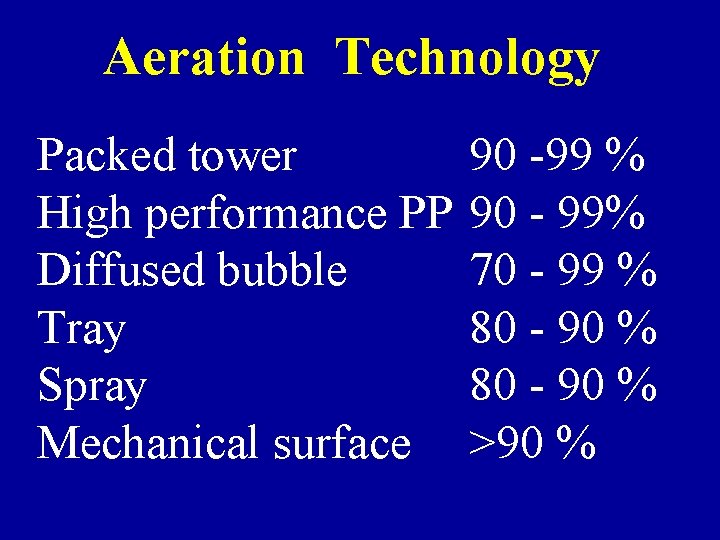
Aeration Technology Packed tower High performance PP Diffused bubble Tray Spray Mechanical surface 90 -99 % 90 - 99% 70 - 99 % 80 - 90 % >90 %

GAC Technology Very Small Systems/ POU/POE GAC 80 - 99 % High EBCT requirements Potential radiation exposure problems Potential waste disposal problems

Radium Ra 224 Thorium series Alpha emitter Half life of 3. 6 days Ra 226 Uranium series Alpha emitter Half life of 1620 years

Radium Ra 228 Thorium series Beta emitter Half life of 6. 7 years

Radium Cation Ra+2 Naturally occurring ground water contaminant Current MCL - 5 p. Ci/L (Ra 226 + Ra 228)

Radium Chemistry is similar to calcium and magnesium (hardness elements)

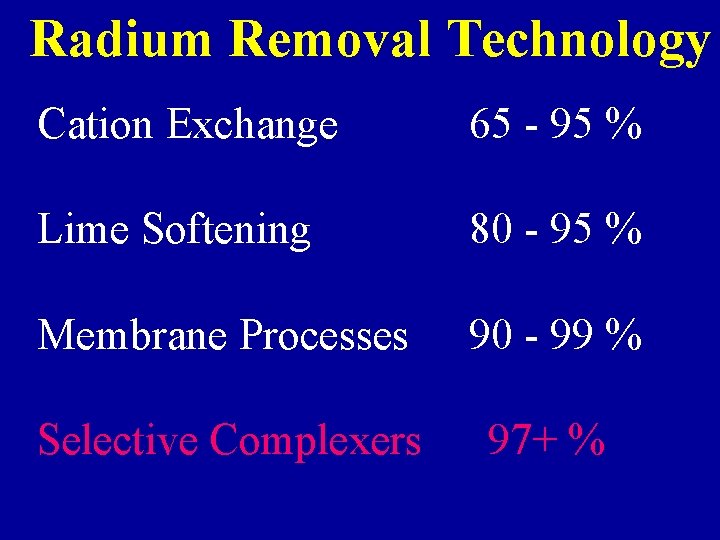
Radium Removal Technology Cation Exchange 65 - 95 % Lime Softening 80 - 95 % Membrane Processes 90 - 99 % Selective Complexers 97+ %

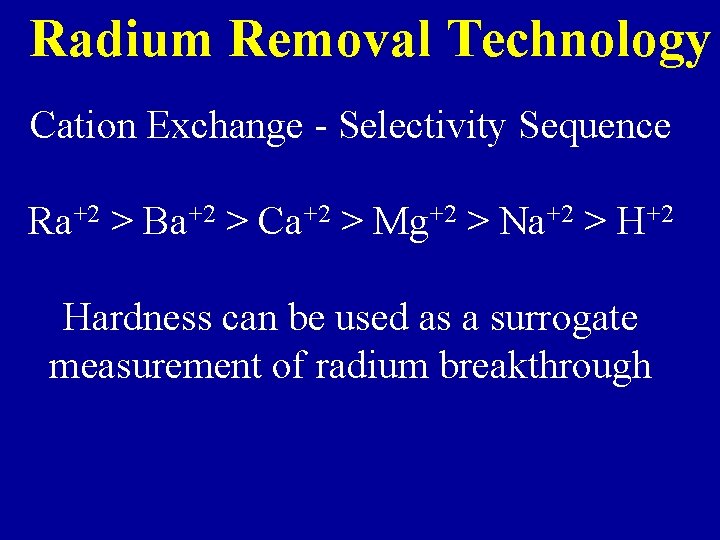
Radium Removal Technology Cation Exchange - Selectivity Sequence Ra+2 > Ba+2 > Ca+2 > Mg+2 > Na+2 > H+2 Hardness can be used as a surrogate measurement of radium breakthrough

Uranium U 238 Uranium series Alpha emitter Half life of 4. 5 x 109 years U 234 Uranium series Alpha emitter Half life of 2. 5 x 105 years

Uranium U 235 Actinium series Alpha emitter Half life of 7. 1 x 106 years

Uranium Cation/Anion/Neutral depending on p. H Naturally occurring ground water contaminant Current MCL - none Proposed MCL in 1991 20 ug/L 30 p. Ci/L

Uranium in Water Chemical Forms p. H < 2. 5 Cation p. H < 2. 5 - 7 Neutral - UO 2(CO 3)0 p. H Anion 7 - 10 - UO 2+ - UO 2(CO 3)-2 - UO 2(CO 3)-4

Uranium Removal Technology Coagulation/Filtration Lime softening Anion Exchange Activated Alumina Membrane processes 80 - 95 % 85 - 99 % 90 - 99 %

Uranium Removal Technology Anion Exchange - High U capacity Treat 10 k -100 k bed volumes Capacity sulfate dependent

Uranium + Radium Removal Technology Cation /Anion Exchange System Ra 100 -1500 BVs U 10 k -100 k BVs Adjust amount of cation / anion resin Optimum mixture - 10 % anion 90 % cation

Gross Alpha, Beta Particle & Photon Emiters MCLs Gross alpha - 15 p. Ci/L (including Ra 226) Beta particle & photon emitters - 4 mrem/year

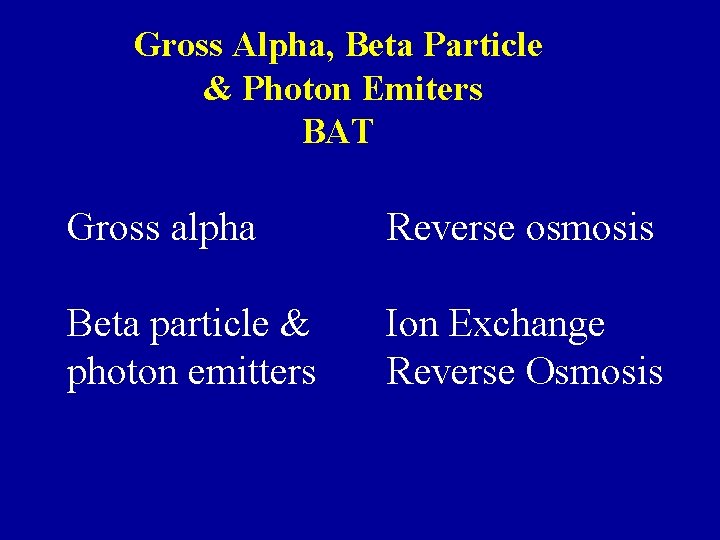
Gross Alpha, Beta Particle & Photon Emiters BAT Gross alpha Reverse osmosis Beta particle & photon emitters Ion Exchange Reverse Osmosis

SUMMARY • Radon, radium & uranium are naturally occurring contaminants usually occurring in ground water.

SUMMARY - RADON • Aeration and GAC are effective treatment technologies for radon. • Of the two technologies, only aeration will be listed as a BAT and likely be the technology of choice in almost all cases. • GAC will likely be considered for only very small systems and for POU/POE.

SUMMARY - RADIUM • All technologies effective for hardness removal are generally effective for radium removal. • Cation exchange, lime softening and reverse osmosis are the technologies currently being applied for radium removal.

SUMMARY - URANIUM • Most conventional technologies have some capability for uranium removal. • Anion exchange has been successfully applied for uranium removal from small ground water systems.

Tom Sorg USEPA Cincinnati, OH 45268 513 -569 -7370 sorg. thomas@epa. gov