Measuring Radon in Residential Properties Training Program Developed



- Slides: 14

Measuring Radon in Residential Properties Training Program Developed by the Healthy Environments for Children Initiative at the University of Connecticut for the Connecticut Department of Public Health 2006

Lesson 1 What is radon?

Characteristics of radon • Gas • Colorless • Odorless • Tasteless • Occurs in nature • Radioactive • Harmful to human health 2

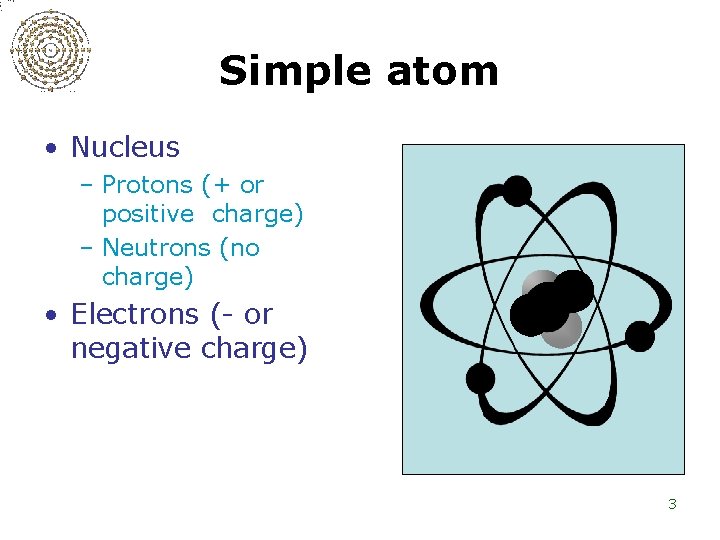
Simple atom • Nucleus – Protons (+ or positive charge) – Neutrons (no charge) • Electrons (- or negative charge) 3

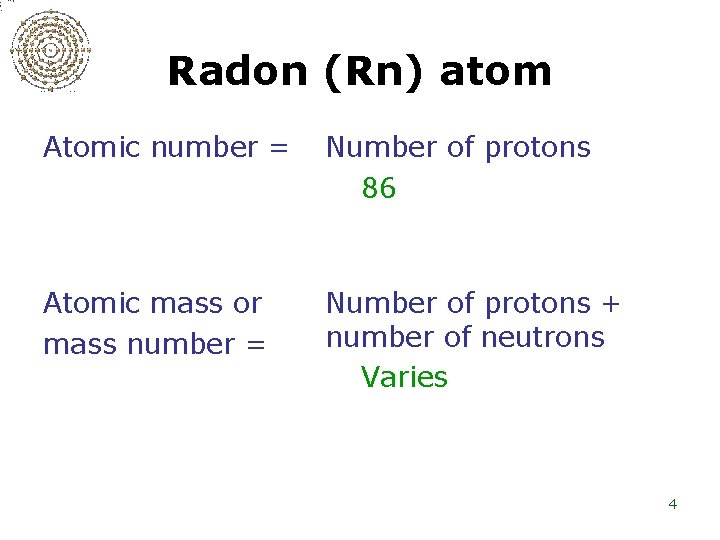
Radon (Rn) atom Atomic number = Number of protons 86 Atomic mass or mass number = Number of protons + number of neutrons Varies 4

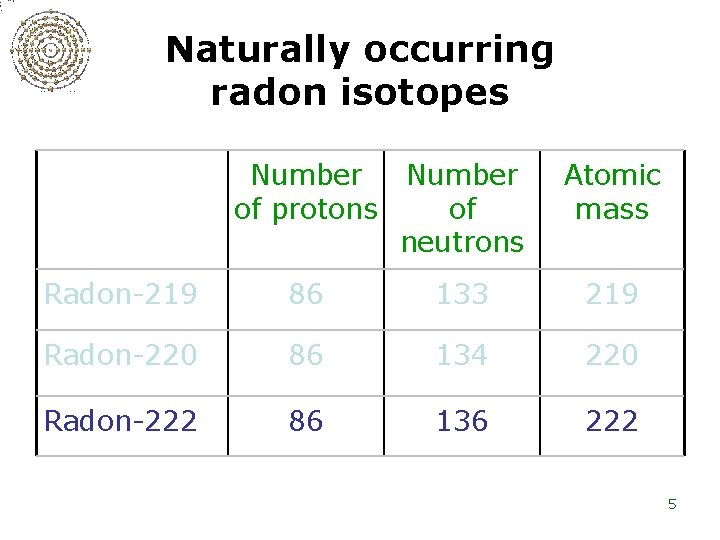
Naturally occurring radon isotopes Number of protons Number of neutrons Atomic mass Radon-219 86 133 219 Radon-220 86 134 220 Radon-222 86 136 222 5

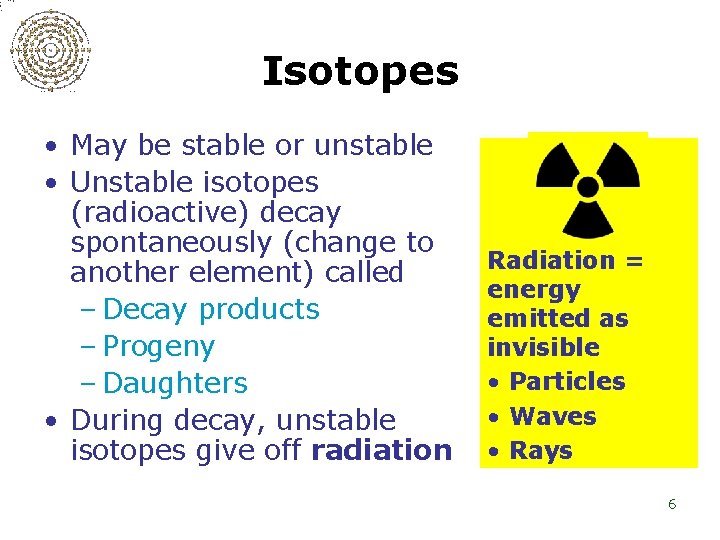
Isotopes • May be stable or unstable • Unstable isotopes (radioactive) decay spontaneously (change to another element) called – Decay products – Progeny – Daughters • During decay, unstable isotopes give off radiation Radiation = energy emitted as invisible • Particles • Waves • Rays 6

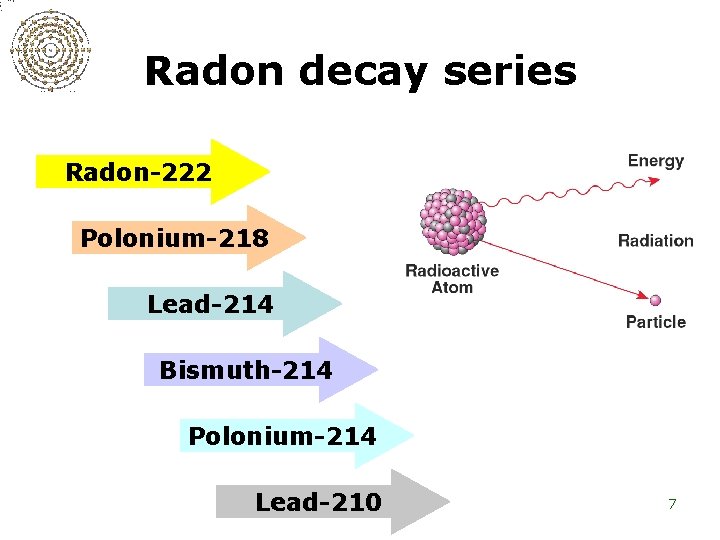
Radon decay series Radon-222 Polonium-218 Lead-214 Bismuth-214 Polonium-214 Lead-210 7

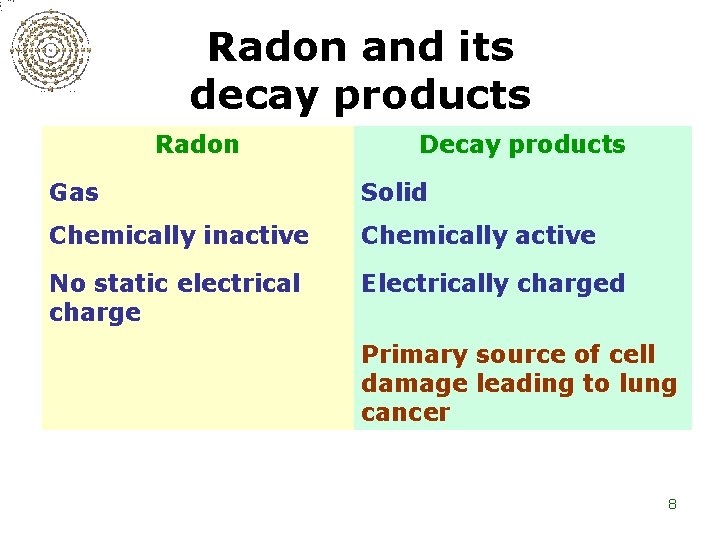
Radon and its decay products Radon Decay products Gas Solid Chemically inactive Chemically active No static electrical charge Electrically charged Primary source of cell damage leading to lung cancer 8

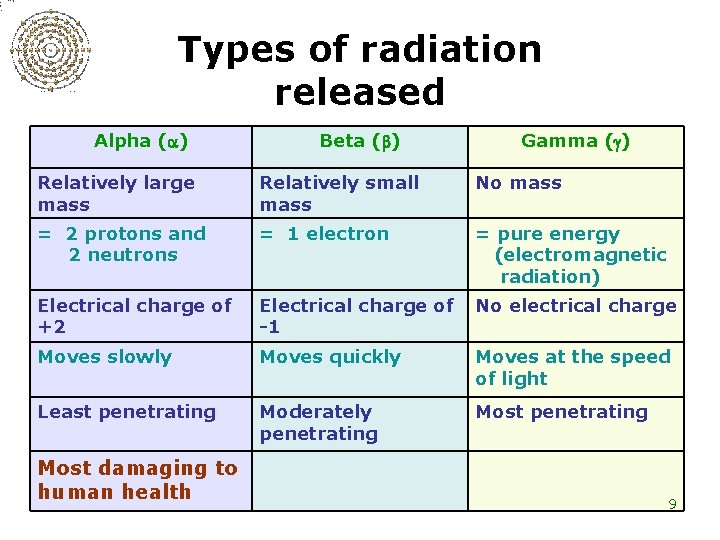
Types of radiation released Alpha ( ) Beta ( ) Gamma ( ) Relatively large mass Relatively small mass No mass = 2 protons and 2 neutrons = 1 electron = pure energy (electromagnetic radiation) Electrical charge of +2 Electrical charge of -1 No electrical charge Moves slowly Moves quickly Moves at the speed of light Least penetrating Moderately penetrating Most damaging to human health 9

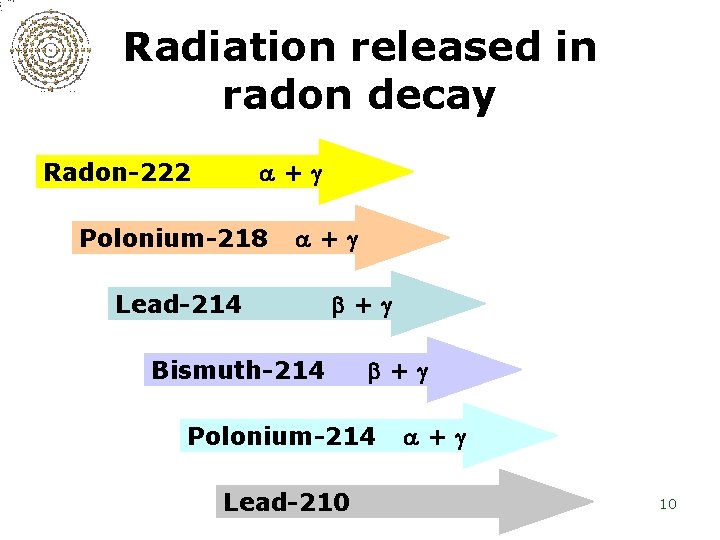
Radiation released in radon decay Radon-222 + Polonium-218 + Lead-214 + Bismuth-214 + Polonium-214 Lead-210 + 10

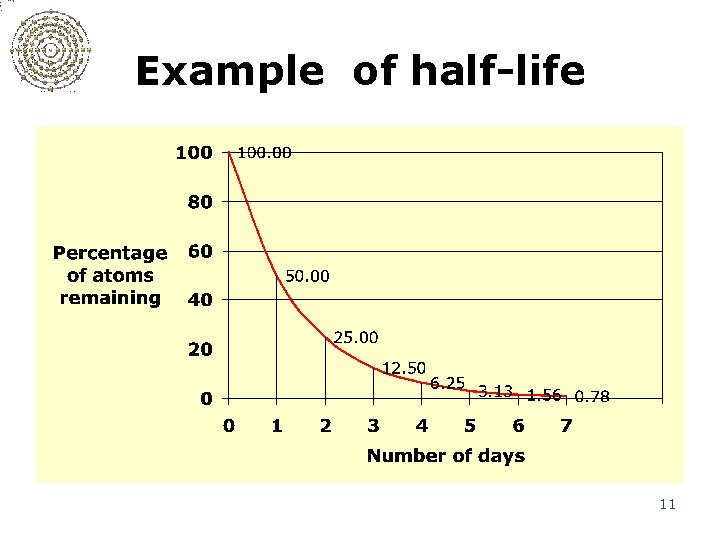
Example of half-life 11

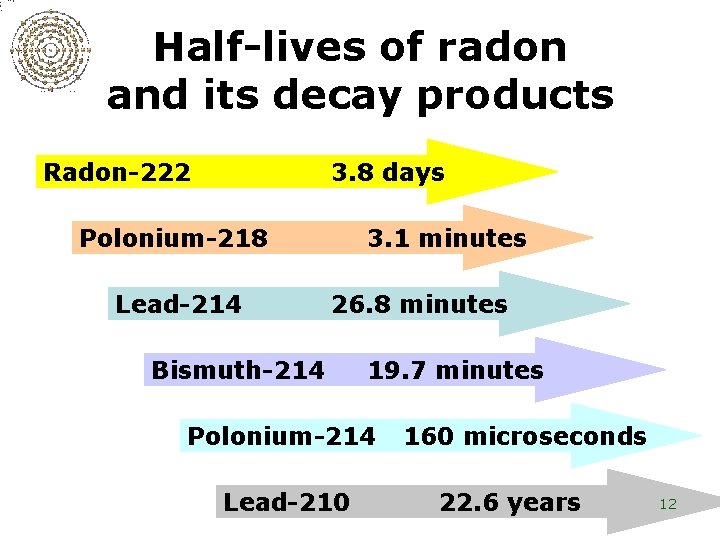
Half-lives of radon and its decay products Radon-222 3. 8 days Polonium-218 Lead-214 3. 1 minutes 26. 8 minutes Bismuth-214 19. 7 minutes Polonium-214 Lead-210 160 microseconds 22. 6 years 12

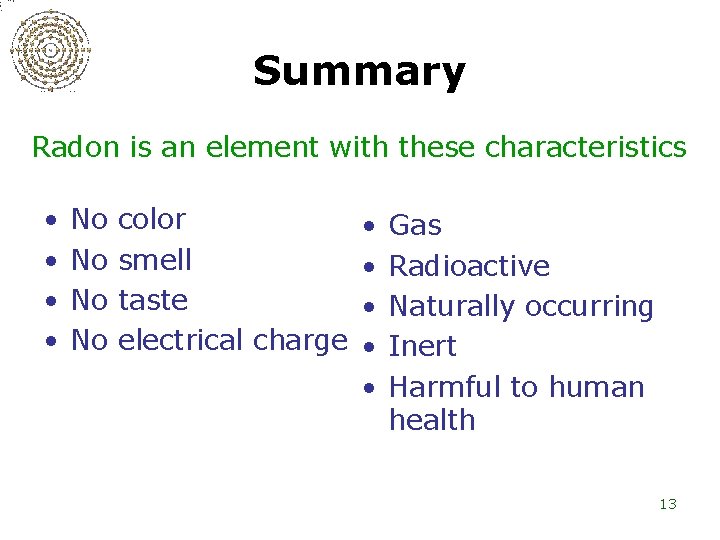
Summary Radon is an element with these characteristics • • No No color • smell • taste • electrical charge • • Gas Radioactive Naturally occurring Inert Harmful to human health 13