Radon Introduction Radon is a colorless and odorless















- Slides: 23

Radon

Introduction Ø Radon is a colorless and odorless gas produced by the decay of radium – 226 Ø Radon after decay produces radioisotopes known as radon daughters Ø Radon progenies (Po-218 and Po-214) are of health concern, as they tend to retain in the lungs causing cancer Ø The upper limit recommended by US EPA for radon is 4 p. Ci/L Ø Radon is found in many states in the USA

Sources

Sources of Radon Ø Sources of radon include v Soil v Rocks beneath or surrounding the building v Water v Building materials v Natural gas Ø Radon from soil moves slowly from the pores of the soil to the surface by diffusion or pressure induced flow Ø Radon enters the building from the cracks and joints in the foundation

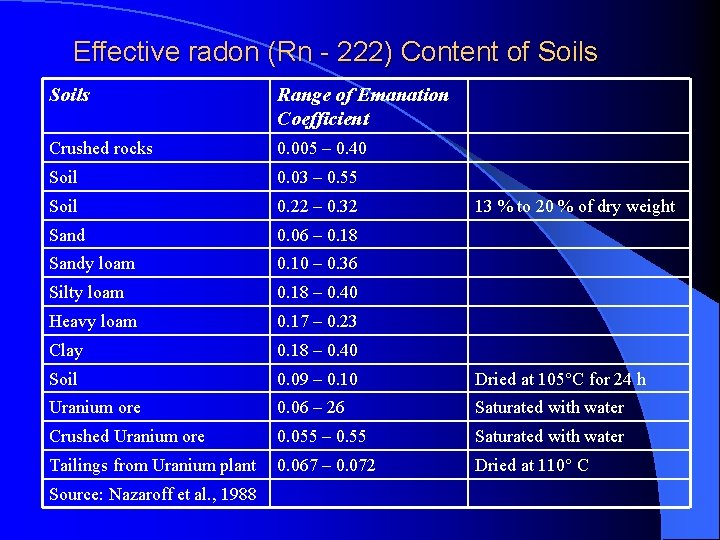
Effective radon (Rn - 222) Content of Soils Range of Emanation Coefficient Crushed rocks 0. 005 – 0. 40 Soil 0. 03 – 0. 55 Soil 0. 22 – 0. 32 Sand 0. 06 – 0. 18 Sandy loam 0. 10 – 0. 36 Silty loam 0. 18 – 0. 40 Heavy loam 0. 17 – 0. 23 Clay 0. 18 – 0. 40 Soil 0. 09 – 0. 10 Dried at 105°C for 24 h Uranium ore 0. 06 – 26 Saturated with water Crushed Uranium ore 0. 055 – 0. 55 Saturated with water Tailings from Uranium plant 0. 067 – 0. 072 Dried at 110° C Source: Nazaroff et al. , 1988 13 % to 20 % of dry weight

Factors affecting transport of Radon to the surface Ø Soil permeability Ø Porosity Ø Water content Ø Temperature Ø Pressure difference between soil and building structure

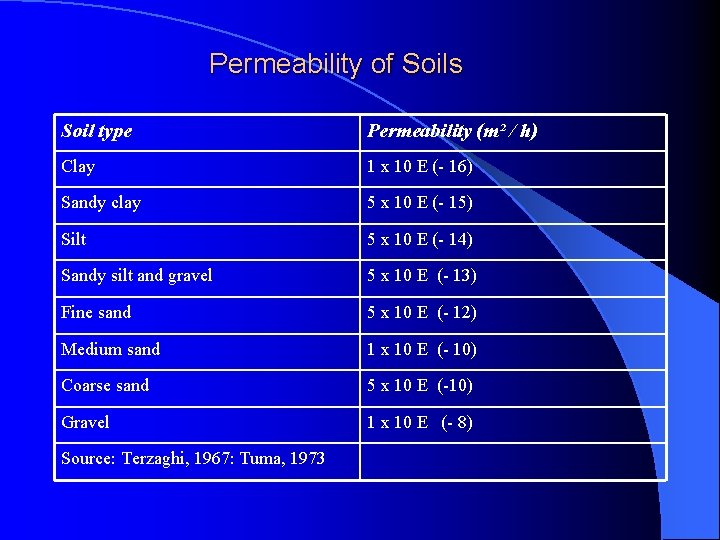
Permeability of Soils Soil type Permeability (m² / h) Clay 1 x 10 E (- 16) Sandy clay 5 x 10 E (- 15) Silt 5 x 10 E (- 14) Sandy silt and gravel 5 x 10 E (- 13) Fine sand 5 x 10 E (- 12) Medium sand 1 x 10 E (- 10) Coarse sand 5 x 10 E (-10) Gravel 1 x 10 E (- 8) Source: Terzaghi, 1967: Tuma, 1973

Sources of Radon Ø Water is also one of the potential sources due to high solubility of radon Ø The transfer of radon from water to air decides its contribution to the indoor concentration Ø Building materials like granite, clay bricks, marble and sandstone are also sources of radon Ø Fly ash from coal-fired power plant is a major source of radon, which is used in concrete and cement

Sampling and Measurement

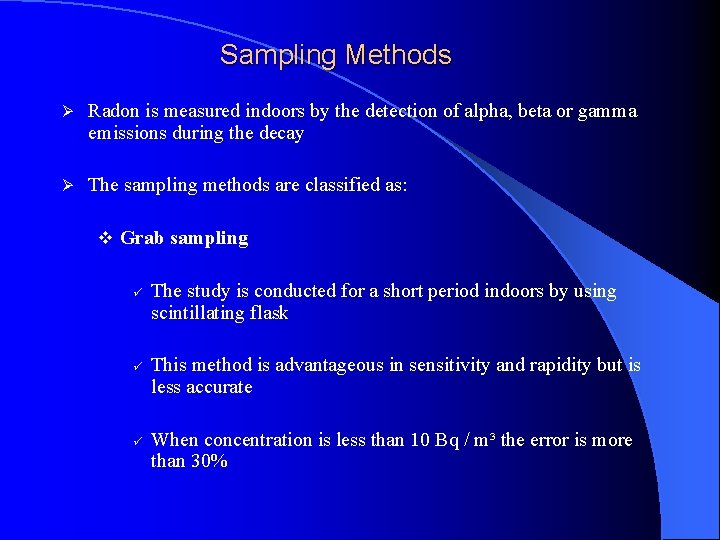
Sampling Methods Ø Radon is measured indoors by the detection of alpha, beta or gamma emissions during the decay Ø The sampling methods are classified as: v Grab sampling ü ü ü The study is conducted for a short period indoors by using scintillating flask This method is advantageous in sensitivity and rapidity but is less accurate When concentration is less than 10 Bq / m³ the error is more than 30%

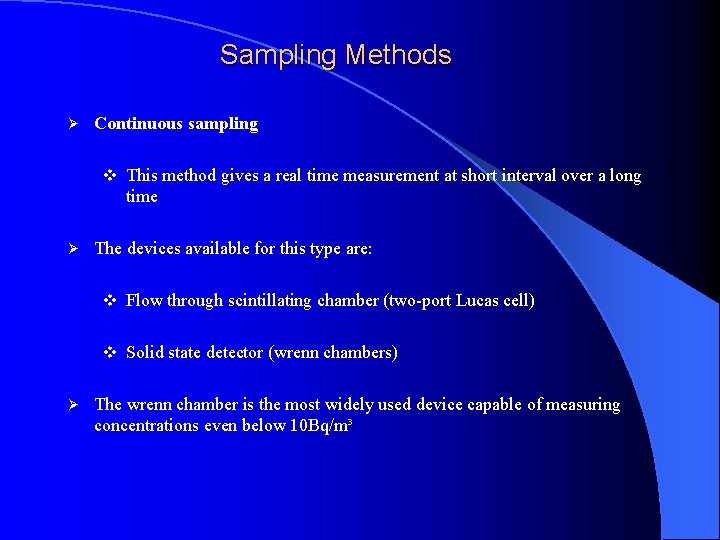
Sampling Methods Ø Continuous sampling v This method gives a real time measurement at short interval over a long time Ø The devices available for this type are: v Flow through scintillating chamber (two-port Lucas cell) v Solid state detector (wrenn chambers) Ø The wrenn chamber is the most widely used device capable of measuring concentrations even below 10 Bq/m³

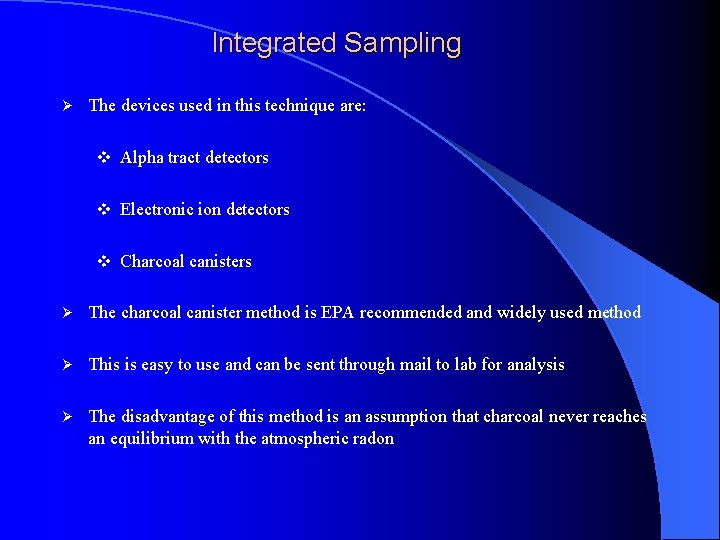
Integrated Sampling Ø The devices used in this technique are: v Alpha tract detectors v Electronic ion detectors v Charcoal canisters Ø The charcoal canister method is EPA recommended and widely used method Ø This is easy to use and can be sent through mail to lab for analysis Ø The disadvantage of this method is an assumption that charcoal never reaches an equilibrium with the atmospheric radon

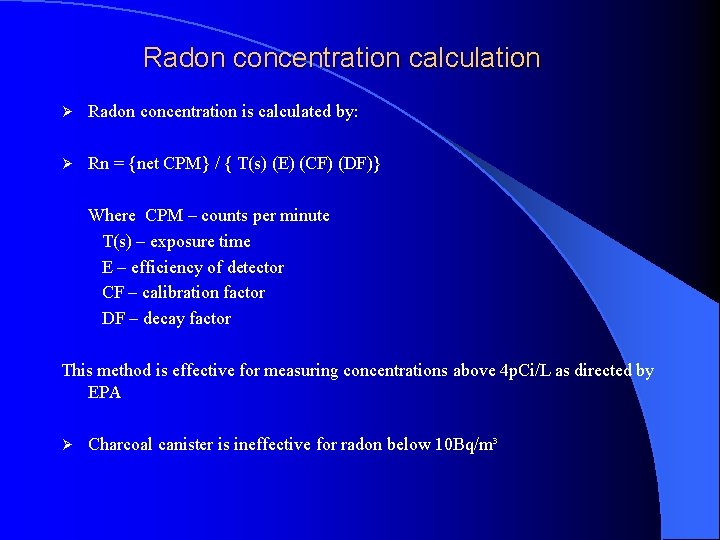
Radon concentration calculation Ø Radon concentration is calculated by: Ø Rn = {net CPM} / { T(s) (E) (CF) (DF)} Where CPM – counts per minute T(s) – exposure time E – efficiency of detector CF – calibration factor DF – decay factor This method is effective for measuring concentrations above 4 p. Ci/L as directed by EPA Ø Charcoal canister is ineffective for radon below 10 Bq/m³

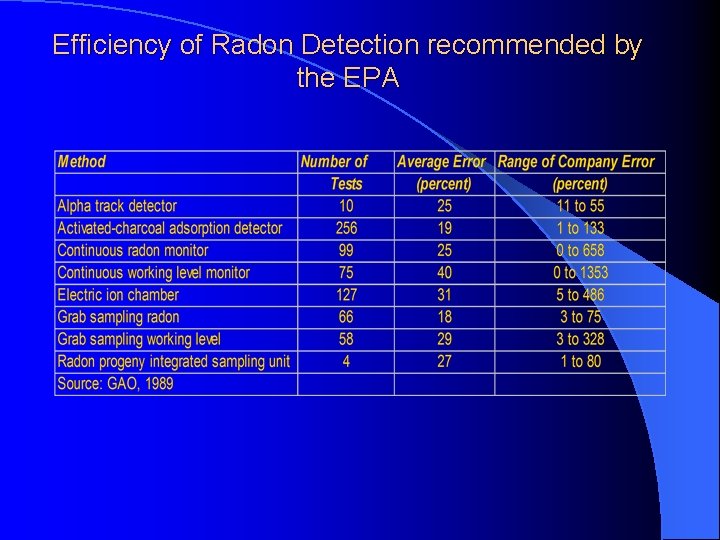
Efficiency of Radon Detection recommended by the EPA

Control Strategies

Source removal Ø Selection of construction sites having low radium content Ø Knowledge of local soil characteristics such as permeability and moisture content Ø Removal and replacement of soil from a perimeter of 3 m from the building foundation Ø The cost for this process is site specific and can range from $5, 000 to $20, 000

New construction considerations Ø Radon concentration can be substantially reduced by new construction techniques Ø Provision of soil gas outlet to the sun slab and crawl spaces Ø Increasing the permeability by placing minimum of 4 inches of aggregate under slab Ø Double barrier approach can be used for slab-on-grade and crawl space construction

Source Control by sealing Entry paths Ø Floor drains and sumps connected to drainage systems Ø Openings around utility lines Ø Hollow concrete block walls Ø Junction between walls and floor and slab Ø Cracks in building materials Ø Exposed soil and rocks having radon Ø Unpaved crawl space

Sealing agents available and their characteristics Ø Caulking agents Ø Paints Ø Membranes Ø Cement-type materials Ø The sealants used should be moisture resistant Ø Paints for walls.

Sunslab ventilation Ø The design of sunslab ventilation is house specific and depends on nature of foundation Ø Fan with a capability to create 50 – 100 Pa is installed on end of the pipe running from the basement Ø This can be made effective by placing multiple collection ports for each wall Ø This is good for old structures, but excessive cracks diminish its effectiveness Ø This is very effective if drain tiles surround the entire house

Basement pressurization and Air cleaning Ø This method is highly effective method if the basement is airtight Ø Over pressurization of the basement drastically reduces the radon concentration below 4 p. Ci / L Ø This method is disadvantageous where there is increased ventilation and excessive windows and doors activity Ø This is one of the ways of reducing the radon concentration Ø During this process the air exchange rates are increased using the HVAC systems Ø Increased ventilation and activated carbon beds can remove the radon gas and its daughter products

Electronic air cleaners and Increased ventilation Ø These cleaners have the capacity of reducing the radon gas and the potential alpha energy concentration (PAEC) by a factor of 2 – 20 Ø After various studies combination of ion generator with ceiling fan produced best results (87% reduction) Ø Another way of decreasing the radon from indoors is plate-out i. e. by pushing the charged progenies to walls or floors and then outdoors Ø Simple, but rather effective technique is to increase the ventilation rate Ø For homes with large crawl spaces mechanical ventilation is adopted to decreasge the radon entry into the building (four fold decrease)

Adsorption Ø The radon adsorption can be another way in reducing its concentration and depends on following factors: v Air flow rates v Radon concentration v Relative humidity Ø Activated carbon is used as adsorbent (having high capacity for radon and minimum interference with moisture and other VOC’s)
 Is pure water colorless
Is pure water colorless Is syngas odorless
Is syngas odorless Ch 28 milady
Ch 28 milady Sentence dictation
Sentence dictation Colorless green ideas sleep furiously
Colorless green ideas sleep furiously Nitrogen is a colorless gas physical or chemical
Nitrogen is a colorless gas physical or chemical Minute colorless anucleate corpuscles found in the blood
Minute colorless anucleate corpuscles found in the blood Colorless gas
Colorless gas Colorless words meaning
Colorless words meaning Colorless green ideas sleep furiously
Colorless green ideas sleep furiously Nail structure and growth
Nail structure and growth List of colorless liquids
List of colorless liquids Klathráty
Klathráty Sinogramma
Sinogramma Retroproiezione tac
Retroproiezione tac Multifamily radon testing
Multifamily radon testing Vilka tre typer av radioaktiv strålning finns det
Vilka tre typer av radioaktiv strålning finns det Radon map maine
Radon map maine Golongan viii
Golongan viii ækvivalent strålingsdosis
ækvivalent strålingsdosis Washington state radon map
Washington state radon map Gazy szlachetne do neonów
Gazy szlachetne do neonów Fontaine a radon
Fontaine a radon Radon transform
Radon transform