TCT 2005 SPIDER Saphenous Vein Graft Protection In






- Slides: 18

TCT 2005 SPIDER Saphenous Vein Graft Protection In a Distal Embolic Protection Randomized Trial Simon R. Dixon MBCh. B, William W. O’Neill MD William Beaumont Hospital, on behalf on the SPIDER Investigators 18 October 2005

SPIDER Objective • To evaluate the safety and efficacy of the SPIDER™/Spide. RX™ Embolic Protection Device during PCI of saphenous vein graft disease

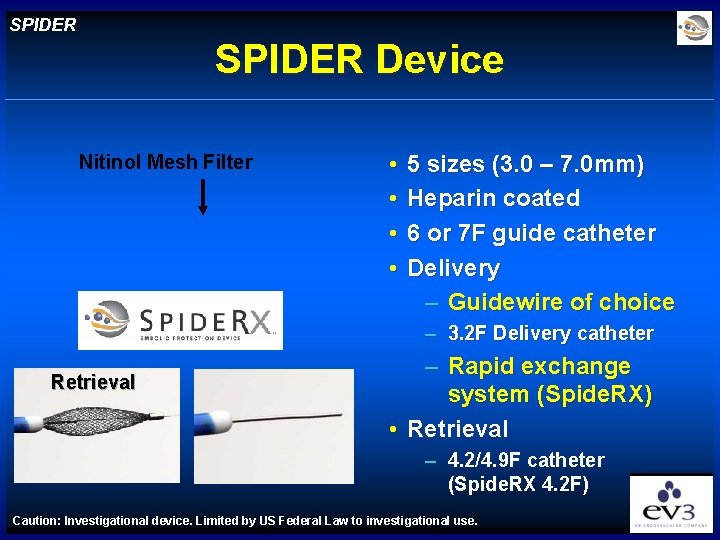
SPIDER Device Nitinol Mesh Filter • • 5 sizes (3. 0 – 7. 0 mm) Heparin coated 6 or 7 F guide catheter Delivery – Guidewire of choice – 3. 2 F Delivery catheter Retrieval – Rapid exchange system (Spide. RX) • Retrieval – 4. 2/4. 9 F catheter (Spide. RX 4. 2 F) Caution: Investigational device. Limited by US Federal Law to investigational use.

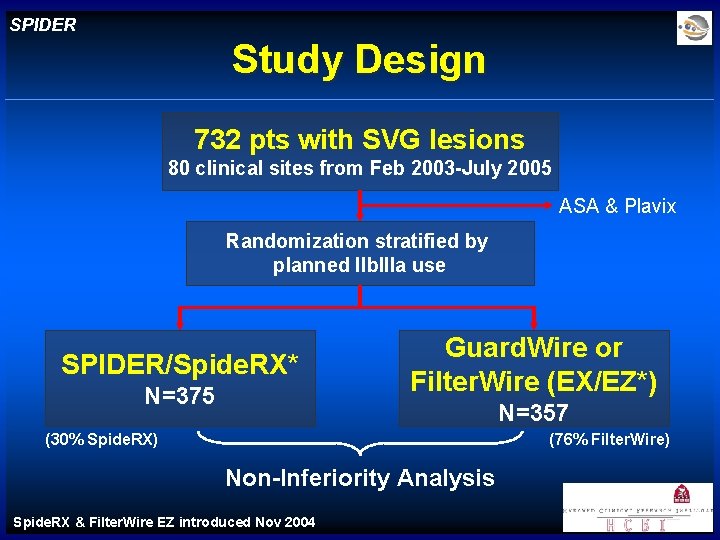
SPIDER Study Design 732 pts with SVG lesions 80 clinical sites from Feb 2003 -July 2005 ASA & Plavix Randomization stratified by planned IIb. IIIa use SPIDER/Spide. RX* N=375 Guard. Wire or Filter. Wire (EX/EZ*) N=357 (30% Spide. RX) (76% Filter. Wire) Non-Inferiority Analysis Spide. RX & Filter. Wire EZ introduced Nov 2004

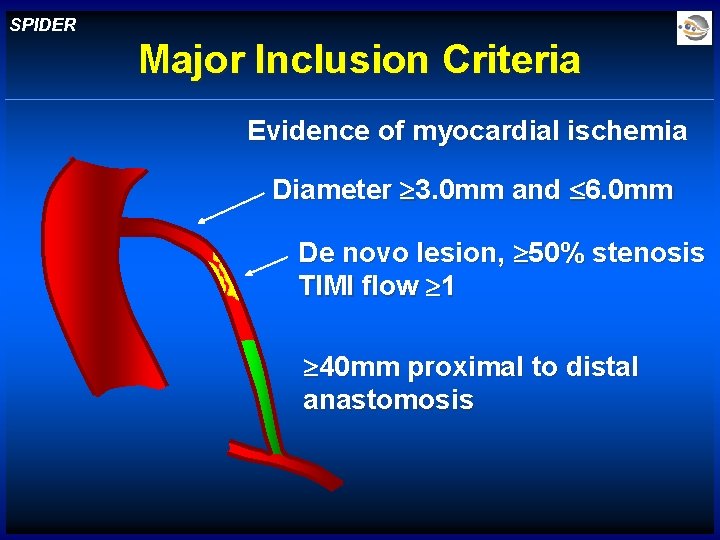
SPIDER Major Inclusion Criteria Evidence of myocardial ischemia Diameter 3. 0 mm and 6. 0 mm De novo lesion, 50% stenosis TIMI flow 1 40 mm proximal to distal anastomosis

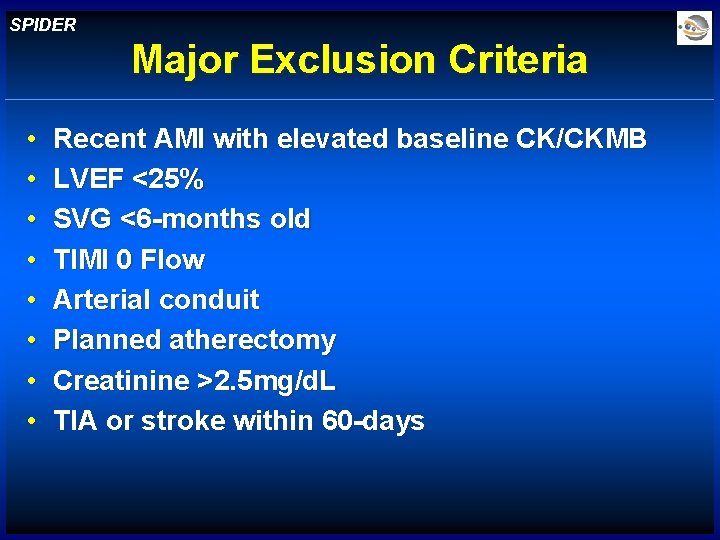
SPIDER Major Exclusion Criteria • • Recent AMI with elevated baseline CK/CKMB LVEF <25% SVG <6 -months old TIMI 0 Flow Arterial conduit Planned atherectomy Creatinine >2. 5 mg/d. L TIA or stroke within 60 -days

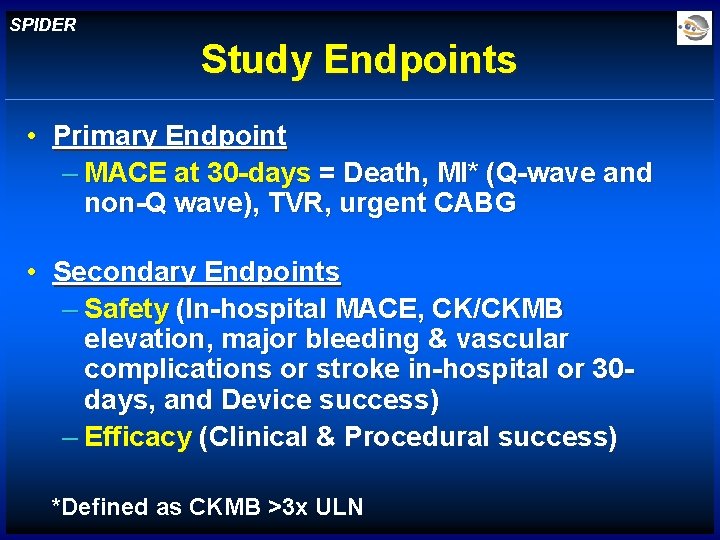
SPIDER Study Endpoints • Primary Endpoint – MACE at 30 -days = Death, MI* (Q-wave and non-Q wave), TVR, urgent CABG • Secondary Endpoints – Safety (In-hospital MACE, CK/CKMB elevation, major bleeding & vascular complications or stroke in-hospital or 30 days, and Device success) – Efficacy (Clinical & Procedural success) *Defined as CKMB >3 x ULN

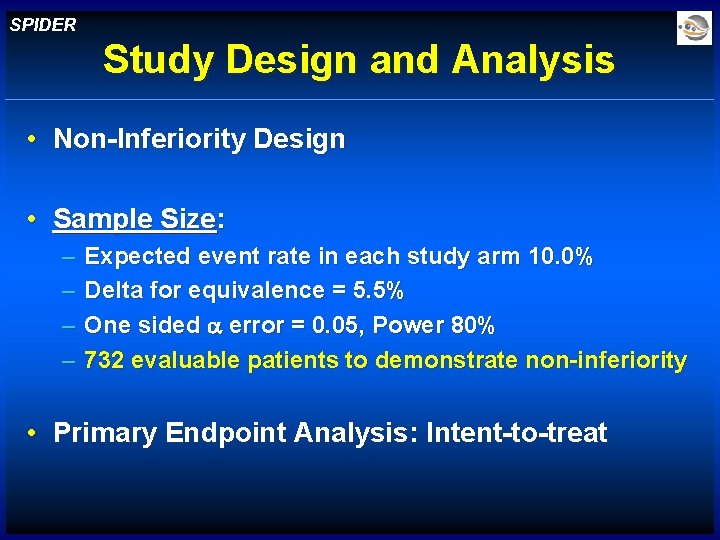
SPIDER Study Design and Analysis • Non-Inferiority Design • Sample Size: – – Expected event rate in each study arm 10. 0% Delta for equivalence = 5. 5% One sided error = 0. 05, Power 80% 732 evaluable patients to demonstrate non-inferiority • Primary Endpoint Analysis: Intent-to-treat

SPIDER Study Organization Principal Investigator William W. O’Neill MD, William Beaumont Hospital Data Management Harvard Clinical Research Institute, Boston, MA. Angiographic Core Lab Brigham & Women’s Hospital, Boston, MA (Jeffrey Popma, MD) HCRI, Boston, MA Peter Zimetbaum MD Bailer Research Group, Lake Hopatcong, NJ ECG Core Lab Study Monitor Sponsor ev 3, Plymouth, MN

SPIDER Top Ten Enrollers • • Munroe Regional Medical Center, Robert Feldman MD William Beaumont Hospital, William O’Neill MD Moses Cone Hospital, Thomas Stuckey MD Peninsula Cardiology Associates, Frank Arena MD St. Vincent Health Center, Jack Smith MD Our Lady of Lourdes Medical Center, Randy Mintz MD Wellmont Holston Valley Medical Center, Christopher Metzger MD • Washington Adventist Hospital, Mark Turco MD • Wake Heart Associates, J. Tift Mann, MD • Tallahassee Memorial Hospital, John Katopodis, MD

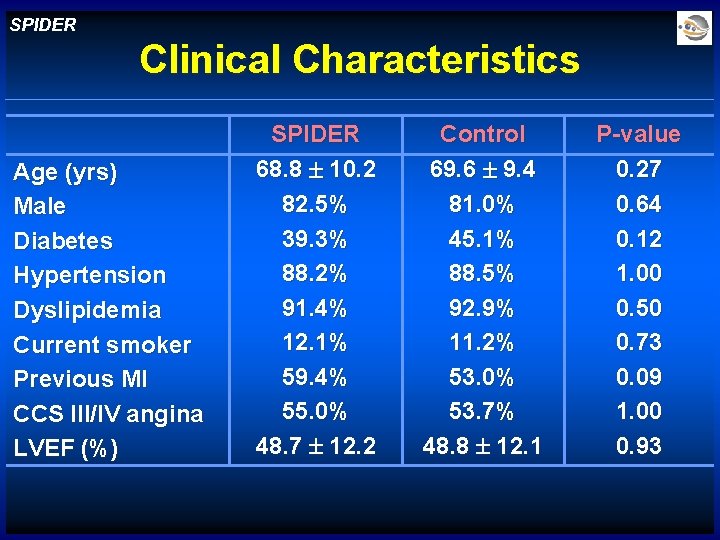
SPIDER Clinical Characteristics Age (yrs) Male Diabetes Hypertension Dyslipidemia Current smoker Previous MI CCS III/IV angina LVEF (%) SPIDER 68. 8 10. 2 82. 5% 39. 3% 88. 2% 91. 4% 12. 1% 59. 4% 55. 0% 48. 7 12. 2 Control 69. 6 9. 4 81. 0% 45. 1% 88. 5% 92. 9% 11. 2% 53. 0% 53. 7% 48. 8 12. 1 P-value 0. 27 0. 64 0. 12 1. 00 0. 50 0. 73 0. 09 1. 00 0. 93

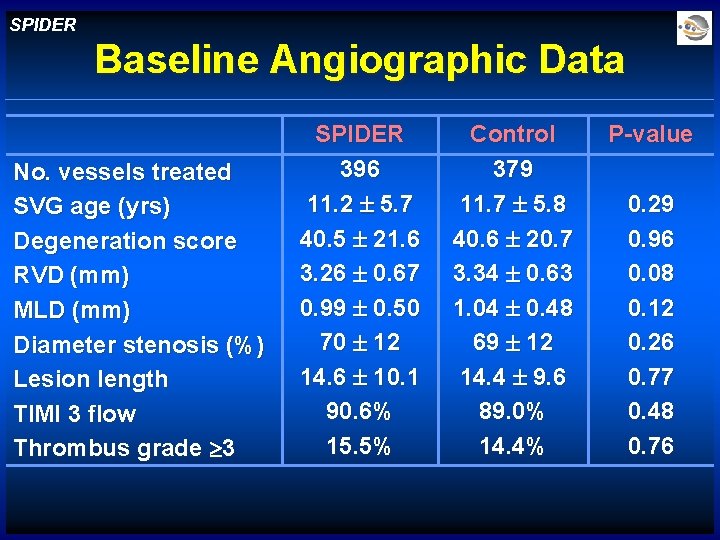
SPIDER Baseline Angiographic Data No. vessels treated SVG age (yrs) Degeneration score RVD (mm) MLD (mm) Diameter stenosis (%) Lesion length TIMI 3 flow Thrombus grade 3 SPIDER 396 11. 2 5. 7 40. 5 21. 6 3. 26 0. 67 0. 99 0. 50 70 12 14. 6 10. 1 90. 6% 15. 5% Control 379 11. 7 5. 8 40. 6 20. 7 3. 34 0. 63 1. 04 0. 48 69 12 14. 4 9. 6 89. 0% 14. 4% P-value 0. 29 0. 96 0. 08 0. 12 0. 26 0. 77 0. 48 0. 76

SPIDER SVG Distribution SPIDER Control N=396 vessels N=379 vessels RCA Circumflex LAD Other P=NS 92. 4% lesions proximal-mid 90. 1% lesions proximal-mid

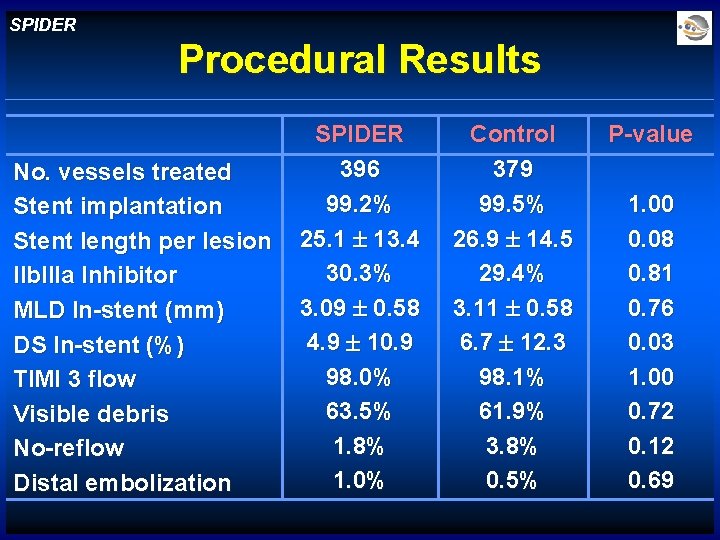
SPIDER Procedural Results No. vessels treated Stent implantation Stent length per lesion IIb. IIIa Inhibitor MLD In-stent (mm) DS In-stent (%) TIMI 3 flow Visible debris No-reflow Distal embolization SPIDER 396 99. 2% 25. 1 13. 4 30. 3% 3. 09 0. 58 4. 9 10. 9 98. 0% 63. 5% 1. 8% 1. 0% Control 379 99. 5% 26. 9 14. 5 29. 4% 3. 11 0. 58 6. 7 12. 3 98. 1% 61. 9% 3. 8% 0. 5% P-value 1. 00 0. 08 0. 81 0. 76 0. 03 1. 00 0. 72 0. 12 0. 69

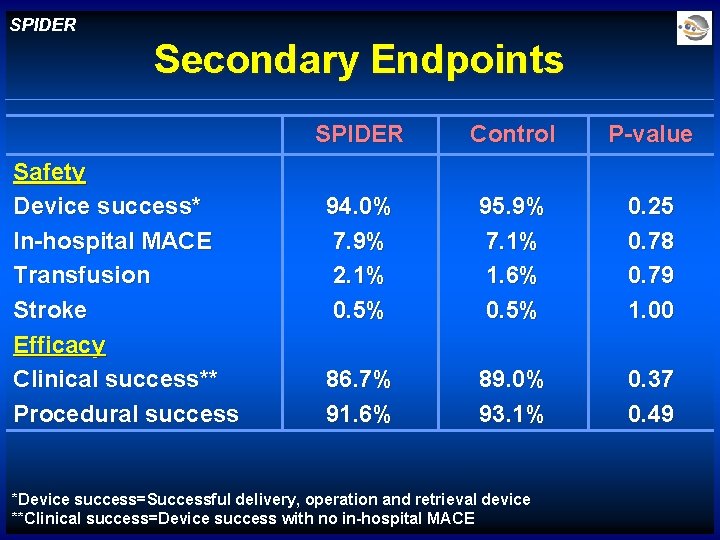
SPIDER Secondary Endpoints Safety Device success* In-hospital MACE Transfusion Stroke Efficacy Clinical success** Procedural success SPIDER Control P-value 94. 0% 7. 9% 2. 1% 0. 5% 95. 9% 7. 1% 1. 6% 0. 5% 0. 25 0. 78 0. 79 1. 00 86. 7% 91. 6% 89. 0% 93. 1% 0. 37 0. 49 *Device success=Successful delivery, operation and retrieval device **Clinical success=Device success with no in-hospital MACE

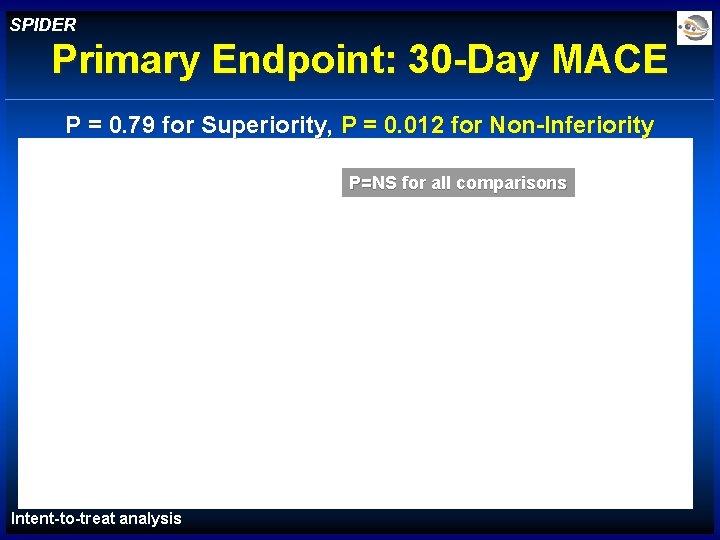
SPIDER Primary Endpoint: 30 -Day MACE P = 0. 79 for Superiority, P = 0. 012 for Non-Inferiority P=NS for all comparisons Intent-to-treat analysis

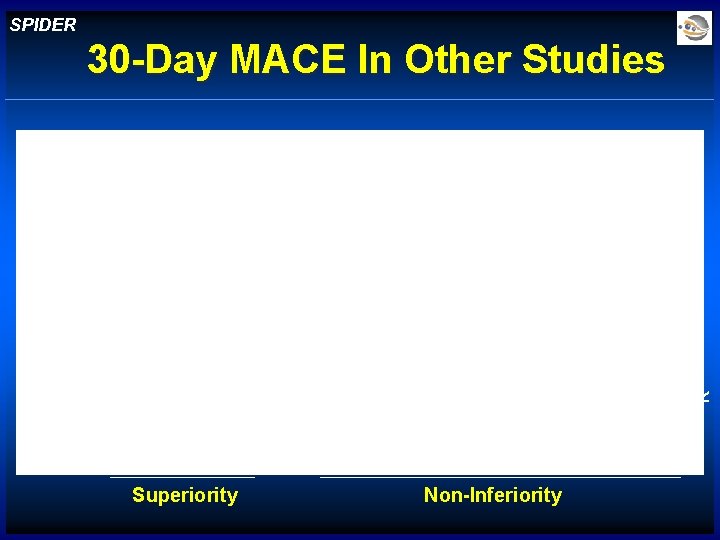
Superiority G ua rd Fil Wi te rre Wi G re ua E rd m Wi bo re sh G iel W d& Tr F i. A W cti G v W S & PI F D W E R T R A P G ua rd Wi re SPIDER 30 -Day MACE In Other Studies Non-Inferiority

SPIDER Conclusion • SPIDER trial demonstrated that distal protection with the SPIDER/Spide. RX Embolic Protection Device during SVG intervention results in a similar rate of MACE at 30 -days and secondary safety endpoints, compared to distal protection with the Guard. Wire and Filter. Wire devices
 Cephalic vein
Cephalic vein Saphenous vein cat
Saphenous vein cat Canine lateral saphenous vein
Canine lateral saphenous vein ชนิดของหลอดเลือด
ชนิดของหลอดเลือด Perforators of leg
Perforators of leg Great and small saphenous vein
Great and small saphenous vein Dorsal arch veins
Dorsal arch veins Great saphenous
Great saphenous Dorsalis pedis vein
Dorsalis pedis vein Dorsal digital vein
Dorsal digital vein Lymphatic and venous drainage
Lymphatic and venous drainage Yellow sac spider map
Yellow sac spider map Tct congress 2018
Tct congress 2018 Tct scan
Tct scan Tct 2013
Tct 2013 Tct
Tct Eye scar
Eye scar Bone graft delivery device
Bone graft delivery device Contoh soal graf sederhana
Contoh soal graf sederhana