Sulfa Drugs Discovery Gerhard Domagk used mice infected
















- Slides: 29

Sulfa Drugs

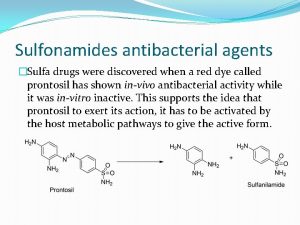
Discovery • Gerhard Domagk used mice infected with haemolytic Streptococcus bacteria as an experimental model and experimented approximately 1000 azo dyes before finding in 1932, a red dye, Prontosil rubrum TM, that was highly active in preventing the death of mice from streptococcal infection. ¨ Prontosil n “Red” -Azo-dye Pre-treatment of bacteria before infection, no effect, but subsequent administration to mice - survival!

n Interestingly, this dye was inactive in vitro against cultures of Streptococcus but clearly active after administration into laboratory animals infected with Gram positive bacteria. n Domagk first treated his daughter, who was very ill with a streptococcal infection, with Prontosil and she made a complete recovery before clinical trials commenced. When clinical trials with Prontosil were published in 1935, it was clear that Prontosil was the first, life-saving antibacterial drug for treating streptococcal infections. n n Ironically, Sulfanilamide was synthesized in 1908 but never tested for antibacterial activity. This discovery prompted a huge interest of this group of drugs, and researchers proceeded to synthesize over 4500 sulfonamides by 1948. Attractive group since they are very cheap.


• • Chemotherapy Selective toxicity Classification by mechanism of action. Resistance.


Mechanism of action • The first clue to the mechanism of antibacterial action of sulfonamides was that para-amino benzoic acid (PABA) reversed the inhibition caused by sulfonamides. • Sulfonamides are analogues of para-amino benzoic acid (PABA), which is an integral part of folic acid. • They competitively inhibit Dihydropteroate Synthase (DHPS) due to its structural resemblance to PABA, the natural substrate for the enzyme & • Sulfonamides cause the production of defective products because it is incorporated in the place of PABA during the production of tetrahydrofolic acid. • Competitive inhibitors of PABA • Bacteriostatic Preventing folate coenzyme synthesis. No deoxythymidine for DNA synthesis is produce

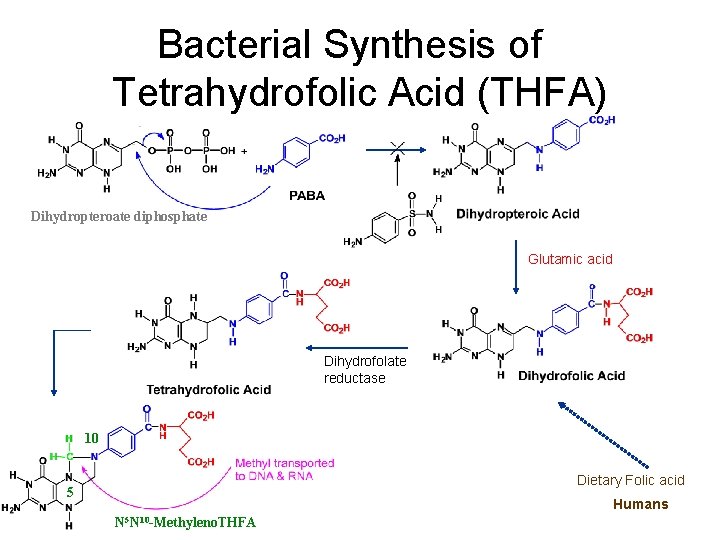
Bacterial Synthesis of Tetrahydrofolic Acid (THFA) Dihydropteroate diphosphate Glutamic acid Dihydrofolate reductase 10 Dietary Folic acid 5 Humans N 5 N 10 -Methyleno. THFA

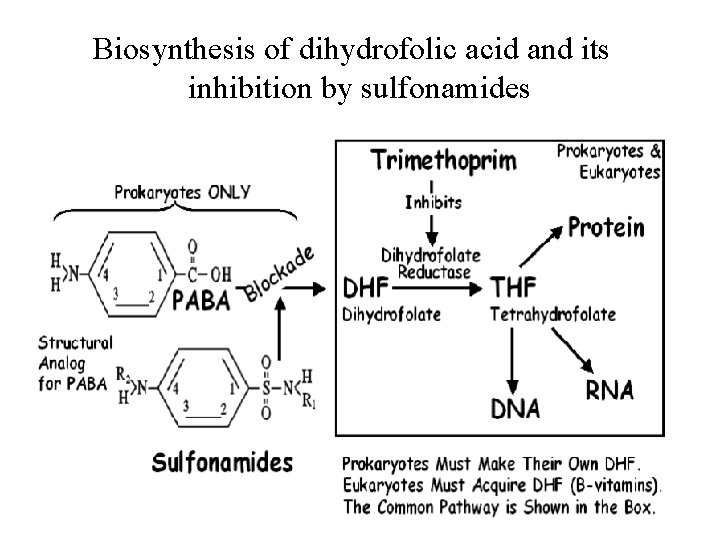
Biosynthesis of dihydrofolic acid and its inhibition by sulfonamides





A number of drugs interfere with the biosynthesis of folic acid and tetrahydrofolate: • Dihydrofolate reductase inhibitors (such as trimethoprim and pyrimethamine). • Sulfonamides (competitive inhibitors of PABA in the reactions of dihydropteroate synthetase. • In order for Sulfonamides to inhibit DHPS, they must first be able to get inside of Bacterial cells. This is accomplished by lipophilicity of Sulfonamides due to the presence of the benzene ring that allows sulfonamides to partition through bacterial cell walls.

Selective toxicity Folic acid is a vitamin for humans : we don’t make it? ? ? • Therefore, no dihydropteroate synthetase • While bacteria lack folic uptake system since they synthesize it • Thus, a selective anti-bacterial agent!

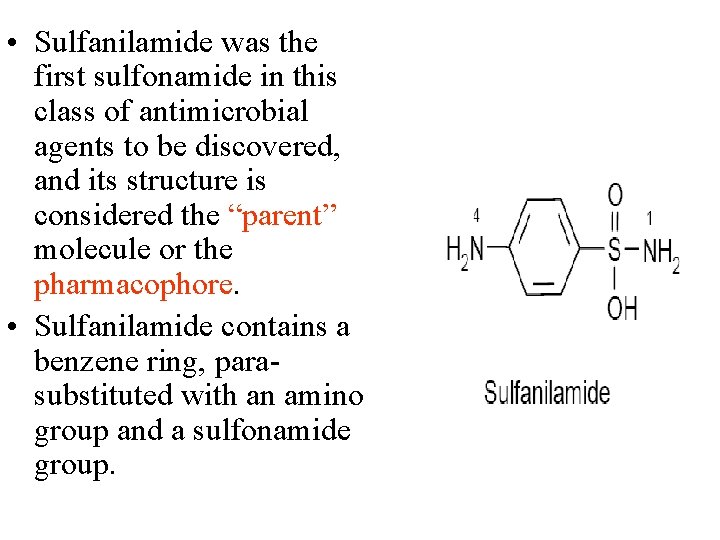
• Sulfanilamide was the first sulfonamide in this class of antimicrobial agents to be discovered, and its structure is considered the “parent” molecule or the pharmacophore. • Sulfanilamide contains a benzene ring, parasubstituted with an amino group and a sulfonamide group.

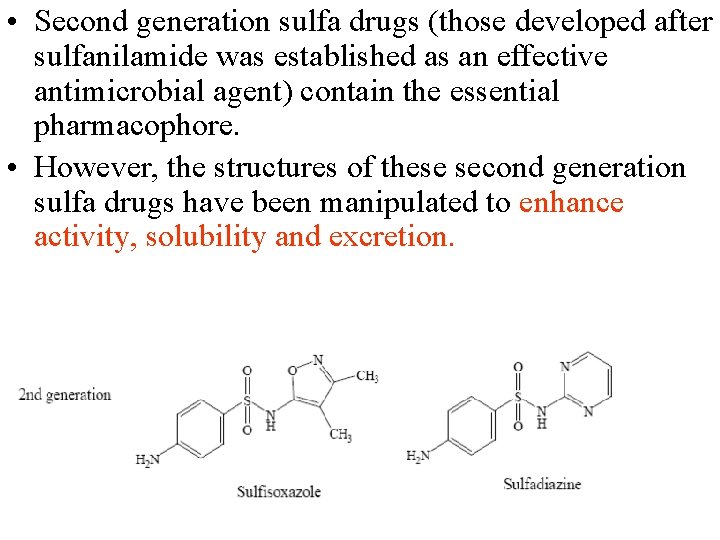
• Second generation sulfa drugs (those developed after sulfanilamide was established as an effective antimicrobial agent) contain the essential pharmacophore. • However, the structures of these second generation sulfa drugs have been manipulated to enhance activity, solubility and excretion.

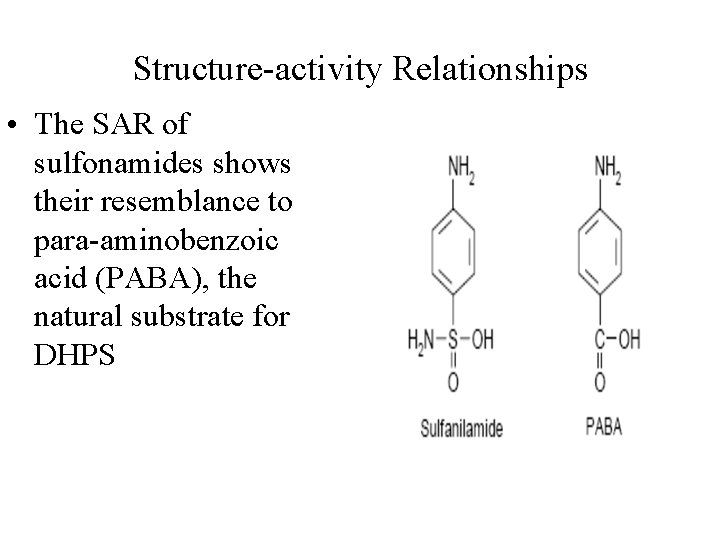
Structure-activity Relationships • The SAR of sulfonamides shows their resemblance to para-aminobenzoic acid (PABA), the natural substrate for DHPS

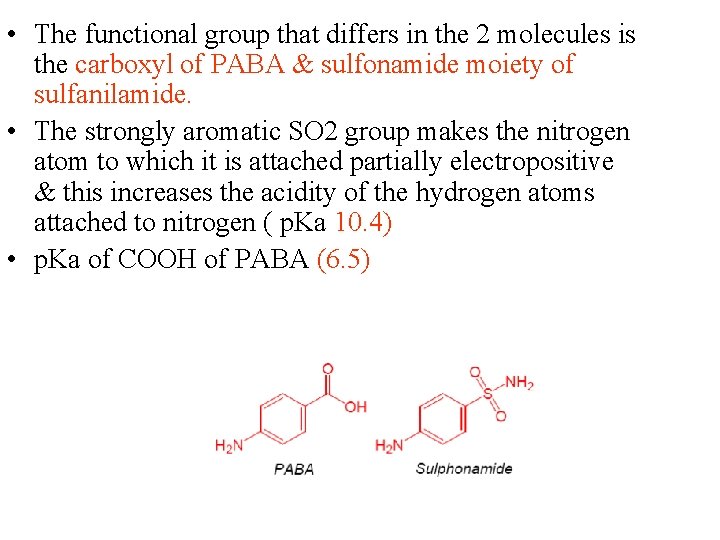
• The functional group that differs in the 2 molecules is the carboxyl of PABA & sulfonamide moiety of sulfanilamide. • The strongly aromatic SO 2 group makes the nitrogen atom to which it is attached partially electropositive & this increases the acidity of the hydrogen atoms attached to nitrogen ( p. Ka 10. 4) • p. Ka of COOH of PABA (6. 5)

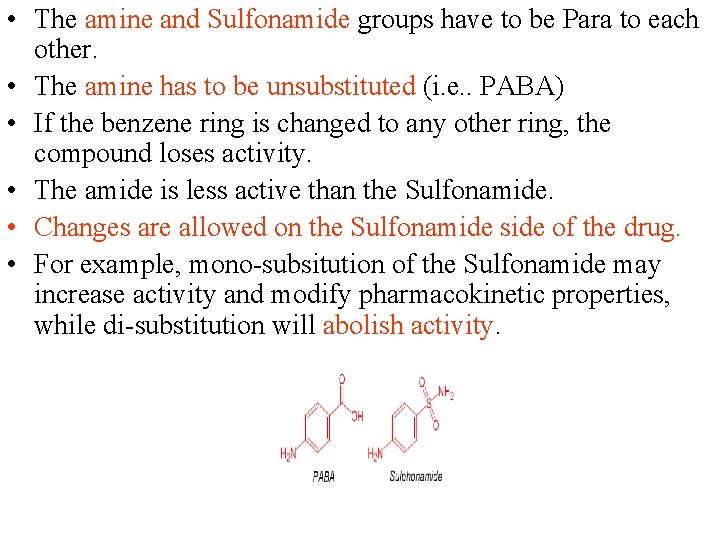
• The amine and Sulfonamide groups have to be Para to each other. • The amine has to be unsubstituted (i. e. . PABA) • If the benzene ring is changed to any other ring, the compound loses activity. • The amide is less active than the Sulfonamide. • Changes are allowed on the Sulfonamide side of the drug. • For example, mono-subsitution of the Sulfonamide may increase activity and modify pharmacokinetic properties, while di-substitution will abolish activity.

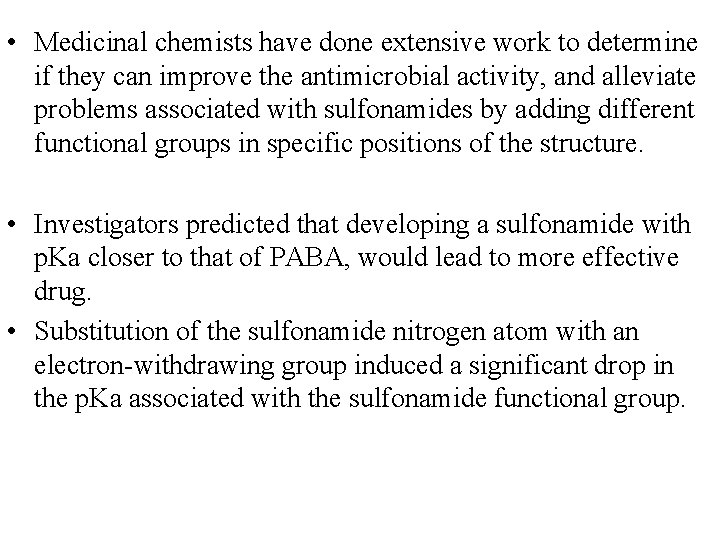
• Medicinal chemists have done extensive work to determine if they can improve the antimicrobial activity, and alleviate problems associated with sulfonamides by adding different functional groups in specific positions of the structure. • Investigators predicted that developing a sulfonamide with p. Ka closer to that of PABA, would lead to more effective drug. • Substitution of the sulfonamide nitrogen atom with an electron-withdrawing group induced a significant drop in the p. Ka associated with the sulfonamide functional group.

• Dropping the p. Ka of the sulfonamide functional group also produced an additional advantage. • The high p. Kas of the earliest sulfonamides tend to have poor solubility, particularly in urine ( p. H ~ 6. 0). • Which lead to precipitation of these compounds from urine, resulting in damage of the kidneys. • Sulfonamides with lower p. Ka are ionized at this p. H, and their water solubility is significantly enhanced, and precipitation of these compounds from urine and kidney toxicity is rare for these compounds.

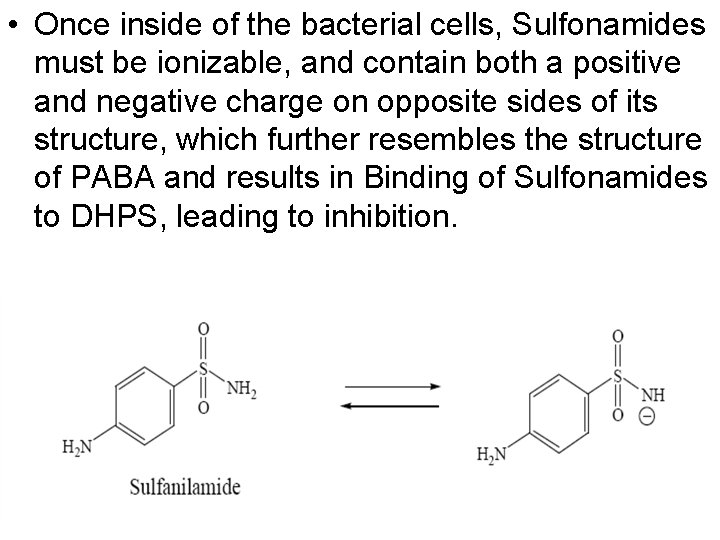
• Once inside of the bacterial cells, Sulfonamides must be ionizable, and contain both a positive and negative charge on opposite sides of its structure, which further resembles the structure of PABA and results in Binding of Sulfonamides to DHPS, leading to inhibition.


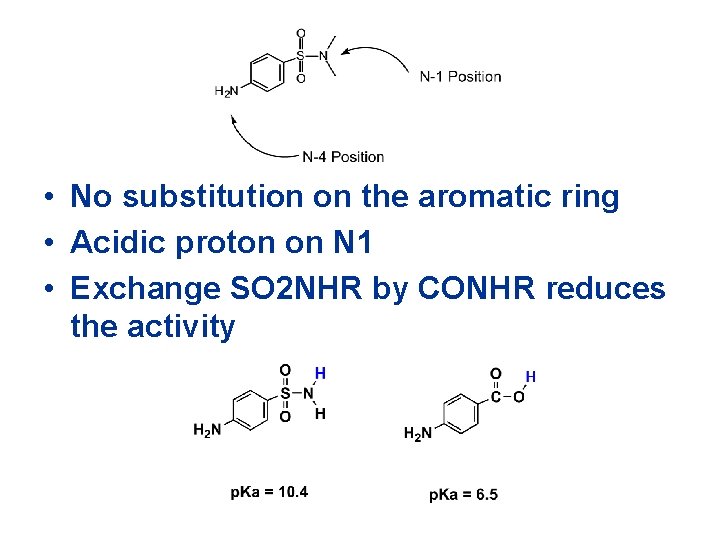
• No substitution on the aromatic ring • Acidic proton on N 1 • Exchange SO 2 NHR by CONHR reduces the activity

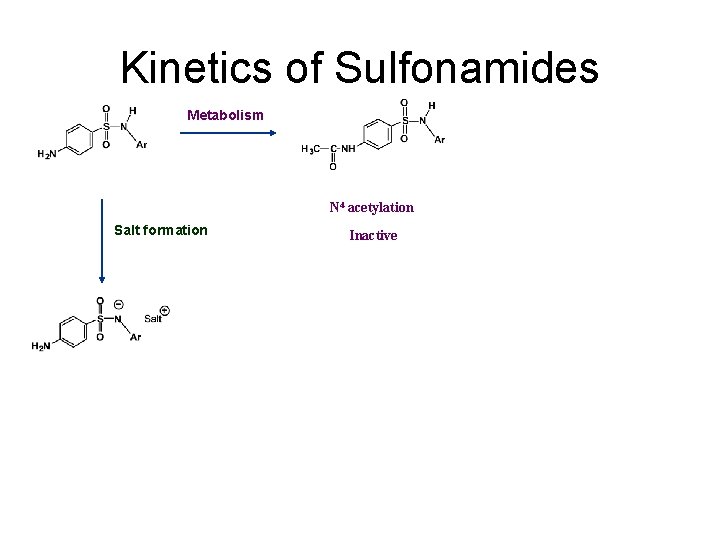
Kinetics of Sulfonamides Metabolism N 4 acetylation Salt formation Inactive

Clinical pharmacokinetics • Most sulfonamides are well absorbed orally and they are widely distributed including to the CNS. • The concentrations in the kidney are the highest. So they are suitable for treating urinary tract infections. • lower solubility in the urine, most sulfonamides and their metabolites easily cause crystalluria, bloody urine, and kidney damage.

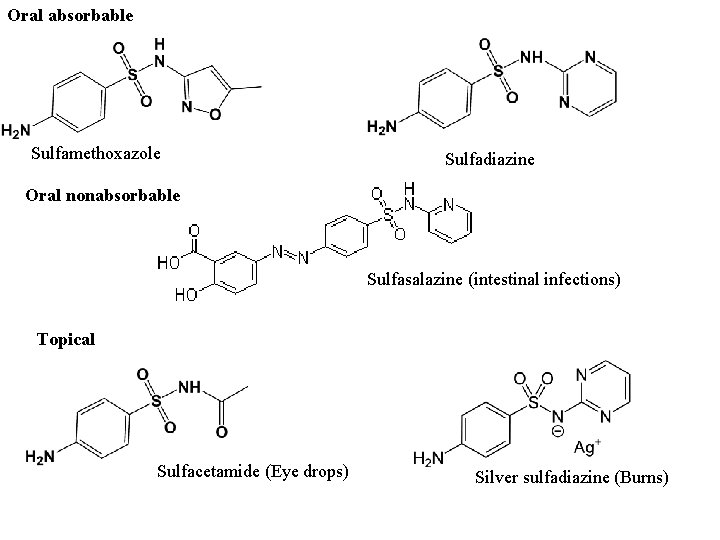
Clinical Uses - Classification of Sulfonamides According to administration ways and absorbed degree in intestinal tract, be divided into 3 groups: ① oral, absorbable: well absorbed in intestinal tract and mainly used to treat general infections. On the basis of their half-lives, also be classified as short-, medium-, or long-acting ones such as sulfisoxazole, sulfamethoxazole and sulfadoxine; ② oral, nonabsorbable: poorly absorbed in intestinal tract and mainly used to treat intestinal tract infections; such as sulfasalazine. ③ topical use: such as SD-Ag and sulfacetamide.

Oral absorbable Sulfamethoxazole Sulfadiazine Oral nonabsorbable Sulfasalazine (intestinal infections) Topical Sulfacetamide (Eye drops) Silver sulfadiazine (Burns)
 Sulfonamides classification
Sulfonamides classification Torilauta 2021
Torilauta 2021 Rubin psychological phases
Rubin psychological phases Difference between liner and varnish
Difference between liner and varnish Postpartum hemorrhage treatment drugs
Postpartum hemorrhage treatment drugs Uterine atony causes
Uterine atony causes Malaria parasites under microscope
Malaria parasites under microscope Malodorous lochia
Malodorous lochia Skos
Skos Walter gerhard
Walter gerhard Gerhard fehringer
Gerhard fehringer Prof dr gerhard schmidt
Prof dr gerhard schmidt Exhibition
Exhibition Gerhard feil
Gerhard feil Gerhard dirmoser
Gerhard dirmoser Gerhard richter abstract painting 1984
Gerhard richter abstract painting 1984 Meritene zusatznahrung
Meritene zusatznahrung Lutz sperling
Lutz sperling Gerhard steger
Gerhard steger Gerhard schedler
Gerhard schedler Gerhard reinecke
Gerhard reinecke Gerhard navratil
Gerhard navratil Gerhard fahnenbruck
Gerhard fahnenbruck Phänomenales feld rogers
Phänomenales feld rogers Gerhard feil
Gerhard feil Dr. gerhard fruhwürth
Dr. gerhard fruhwürth Bw lehrpool
Bw lehrpool Gerhard brodil
Gerhard brodil Arc web northumbria
Arc web northumbria Telecommunication trends
Telecommunication trends