Steps to Success with Multicolor Flow Cytometry Holden








- Slides: 33

Steps to Success with Multicolor Flow Cytometry Holden T. Maecker

Outline 1. Configure your instrument 2. Characterize your instrument 3. Design your panel 4. Optimize settings for your panel 5. Run appropriate controls 6. QC your data Holden Maecker, Flow Cytometry Consulting

Outline 1. Configure your instrument n n Number and type of lasers Number of PMTs per laser Choice of filters and dichroic mirrors These choices will determine: n n What fluorochromes you can use effectively How well certain fluorochrome combinations will perform Holden Maecker, Flow Cytometry Consulting

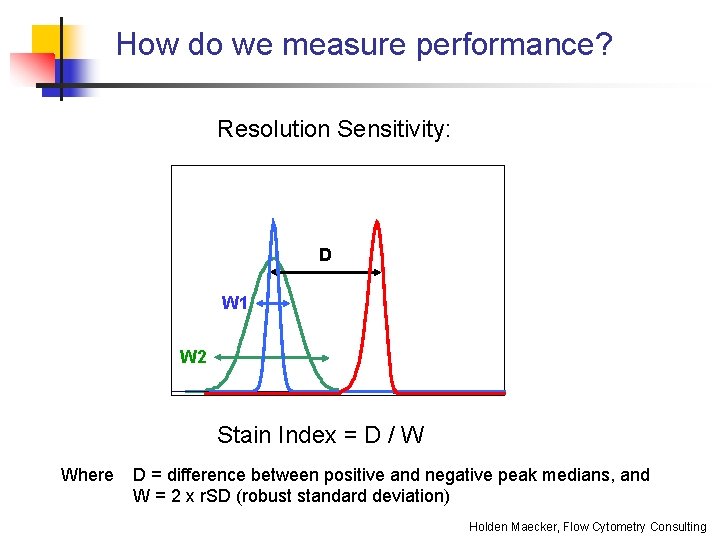
How do we measure performance? Resolution Sensitivity: D W 1 W 2 Stain Index = D / W Where D = difference between positive and negative peak medians, and W = 2 x r. SD (robust standard deviation) Holden Maecker, Flow Cytometry Consulting

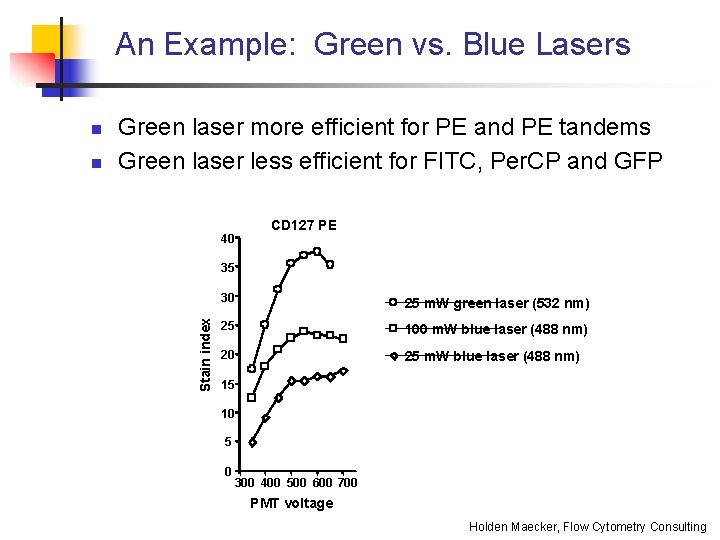
An Example: Green vs. Blue Lasers n Green laser more efficient for PE and PE tandems Green laser less efficient for FITC, Per. CP and GFP 40 CD 127 PE 35 Stain index n 30 25 m. W green laser (532 nm) 25 100 m. W blue laser (488 nm) 20 25 m. W blue laser (488 nm) 15 10 5 0 300 400 500 600 700 PMT voltage Holden Maecker, Flow Cytometry Consulting

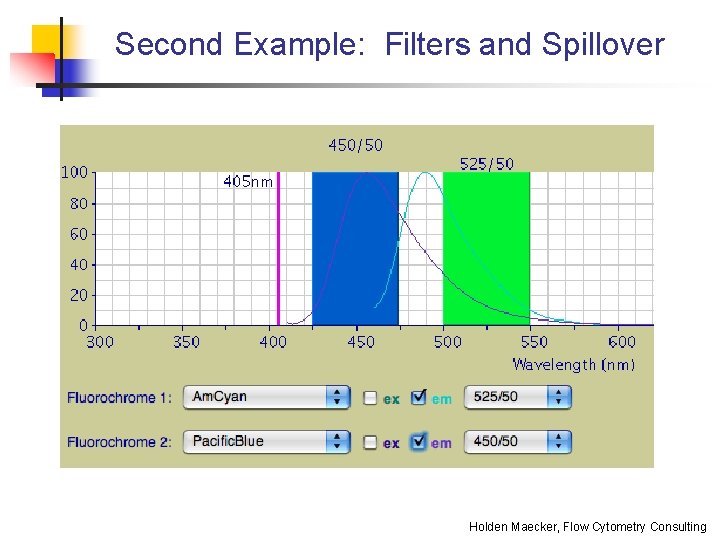
Second Example: Filters and Spillover Holden Maecker, Flow Cytometry Consulting

Outline 2. Characterize your instrument n n n Obtain minimum baseline PMT settings Track performance over time This will allow you to: n n Run the instrument where it is most sensitive Be alert to changes in the instrument that might affect performance Holden Maecker, Flow Cytometry Consulting

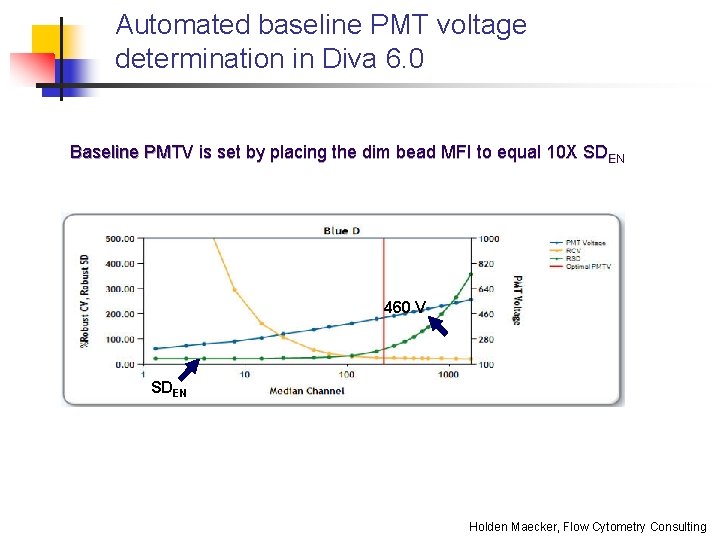
Automated baseline PMT voltage determination in Diva 6. 0 Baseline PMTV is set by placing the dim bead MFI to equal 10 X SDEN 460 V SDEN Holden Maecker, Flow Cytometry Consulting

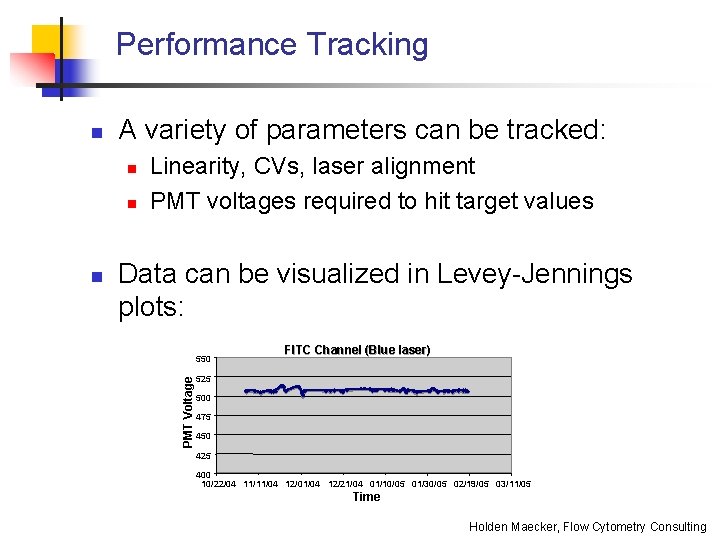
Performance Tracking A variety of parameters can be tracked: n n n Linearity, CVs, laser alignment PMT voltages required to hit target values Data can be visualized in Levey-Jennings plots: 550 PMT Voltage n FITC Channel (Blue laser) 525 500 475 450 425 400 10/22/04 11/11/04 12/01/04 12/21/04 01/10/05 01/30/05 02/19/05 03/11/05 Time Holden Maecker, Flow Cytometry Consulting

Outline 3. Design your panel n n n Reserve brightest fluorochromes for dimmest markers and vice versa Avoid spillover from bright populations into detectors requiring high sensitivity Beware of tandem dye issues Titrate antibodies for best separation This will allow you to: n n Maintain resolution sensitivity where you most need it Avoid artifacts of tandem dye degradation Holden Maecker, Flow Cytometry Consulting

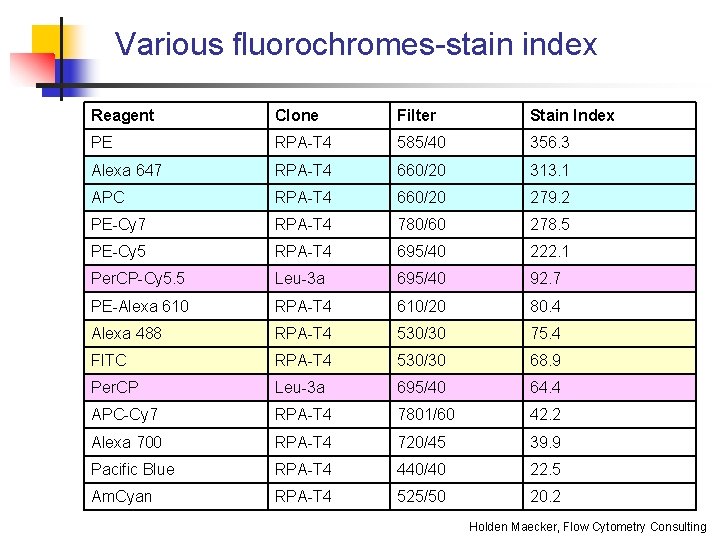
Various fluorochromes-stain index Reagent Clone Filter Stain Index PE RPA-T 4 585/40 356. 3 Alexa 647 RPA-T 4 660/20 313. 1 APC RPA-T 4 660/20 279. 2 PE-Cy 7 RPA-T 4 780/60 278. 5 PE-Cy 5 RPA-T 4 695/40 222. 1 Per. CP-Cy 5. 5 Leu-3 a 695/40 92. 7 PE-Alexa 610 RPA-T 4 610/20 80. 4 Alexa 488 RPA-T 4 530/30 75. 4 FITC RPA-T 4 530/30 68. 9 Per. CP Leu-3 a 695/40 64. 4 APC-Cy 7 RPA-T 4 7801/60 42. 2 Alexa 700 RPA-T 4 720/45 39. 9 Pacific Blue RPA-T 4 440/40 22. 5 Am. Cyan RPA-T 4 525/50 20. 2 Holden Maecker, Flow Cytometry Consulting

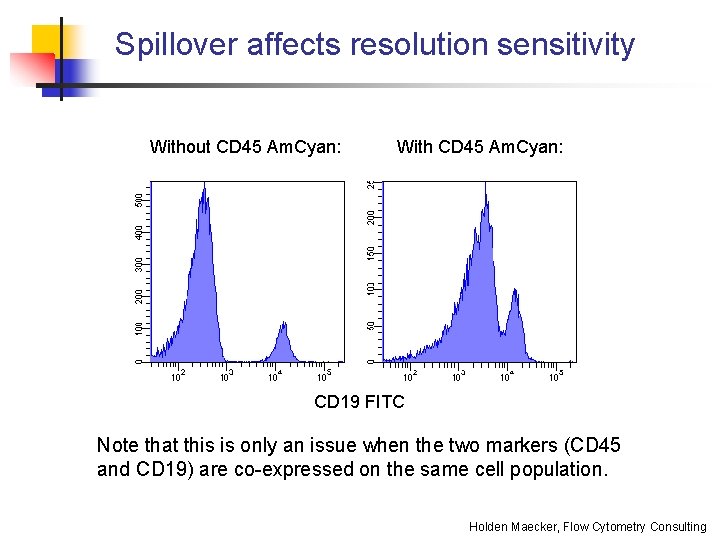
Spillover affects resolution sensitivity Without CD 45 Am. Cyan: With CD 45 Am. Cyan: CD 19 FITC Note that this is only an issue when the two markers (CD 45 and CD 19) are co-expressed on the same cell population. Holden Maecker, Flow Cytometry Consulting

Special requirements of tandem dyes n Compensation requirements for tandem dye conjugates can vary, even between two experiments with the same antibody n n n Degrade with exposure to light, temperature, and fixation Stained cells are most vulnerable Solutions: n n n Minimize exposure to above agents Use BD stabilizing fixative if a final fix is necessary Run experiment-specific compensation Holden Maecker, Flow Cytometry Consulting

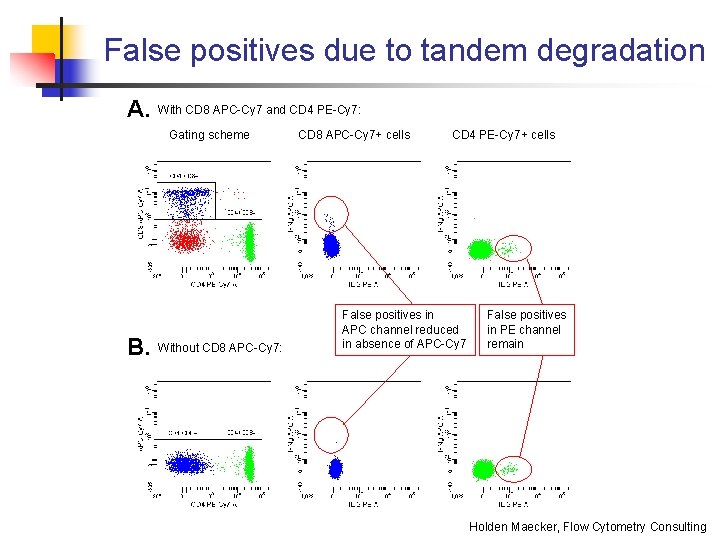
False positives due to tandem degradation A. With CD 8 APC-Cy 7 and CD 4 PE-Cy 7: Gating scheme B. Without CD 8 APC-Cy 7: CD 8 APC-Cy 7+ cells CD 4 PE-Cy 7+ cells False positives in APC channel reduced in absence of APC-Cy 7 False positives in PE channel remain Holden Maecker, Flow Cytometry Consulting

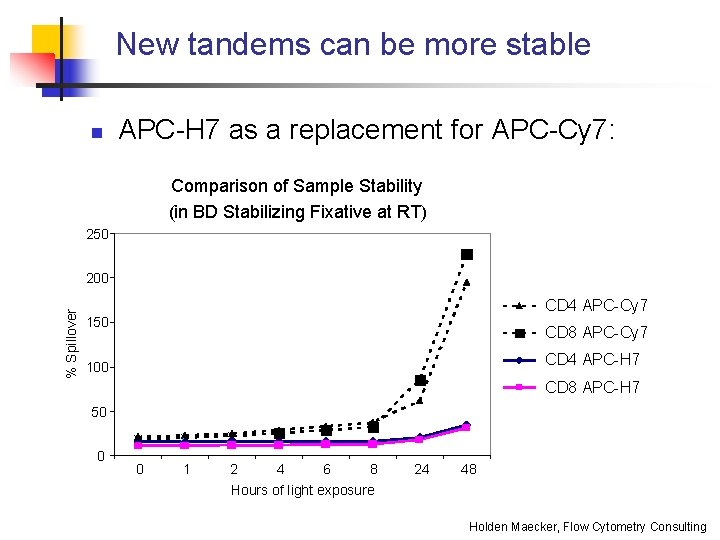
New tandems can be more stable n APC-H 7 as a replacement for APC-Cy 7: Comparison of Sample Stability (in BD Stabilizing Fixative at RT) 250 % Spillover 200 CD 4 APC-Cy 7 150 CD 8 APC-Cy 7 CD 4 APC-H 7 100 CD 8 APC-H 7 50 0 0 1 2 4 6 8 Hours of light exposure 24 48 Holden Maecker, Flow Cytometry Consulting

Antibody titration basics n For most purposes, the main objective is to maximize signal: noise (pos/neg separation) n n n This may occur at less than saturated staining This may or may not be the manufacturer’s recommended titer Titer is affected by: n n Staining volume (e. g. , 100 m. L) Number of cells (not critical up to ~5 x 106) Staining time and temperature (e. g. , 30 min RT) Type of sample (whole blood, PBMC, etc. ) Holden Maecker, Flow Cytometry Consulting

Antibody titration example Holden Maecker, Flow Cytometry Consulting

Outline 4. Optimize settings for your panel n n n Derive experiment-specific PMT settings Run compensation controls for each experiment This will allow you to: n n Use settings most appropriate for your panel Avoid gross errors of compensation Holden Maecker, Flow Cytometry Consulting

Experiment-specific setup for a new panel 1. Set voltages to achieve baseline target values 2. Run single-stained Comp. Beads to see if each bead is at least 2 x brighter in its primary detector vs. other detectors • If not, increase voltage in the primary detector (beware: potential reagent problem) 3. Run fully-stained cells and: • • Decrease voltages for any detectors where events are off-scale Increase voltages for any detectors where low-end resolution is poor (theoretically should not be necessary) 4. Re-run single-stained Comp. Beads and calculate compensation 5. Re-run fully-stained cells and repeat step 3 (if further changes made, re-run compensation) 6. Save experiment-specific settings as target values 7. Run samples Holden Maecker, Flow Cytometry Consulting

Experiment-specific setup for existing panel n n n Set voltages to achieve experiment-specific target channels Run single-stained Comp. Beads and calculate compensation Run samples Holden Maecker, Flow Cytometry Consulting

Outline 5. Run appropriate controls n n Instrument setup controls (e. g. , Comp. Beads) Gating controls (e. g. , FMO) Biological controls (e. g. , unstimulated samples, healthy donors) This will allow you to: n n n Obtain consistent setup and compensation Gate problem markers reproducibly Make appropriate biological comparisons and conclusions Holden Maecker, Flow Cytometry Consulting

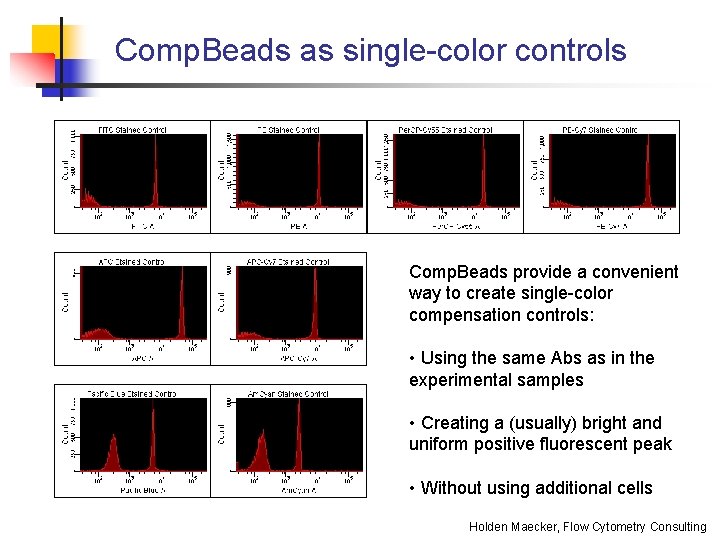
Comp. Beads as single-color controls Comp. Beads provide a convenient way to create single-color compensation controls: • Using the same Abs as in the experimental samples • Creating a (usually) bright and uniform positive fluorescent peak • Without using additional cells Holden Maecker, Flow Cytometry Consulting

Frequent compensation questions n Do I need to use the same antibody for compensation as I use in the experiment? n n Are capture beads better than cells for compensation? n n Yes, for certain tandem dyes (e. g. , PE-Cy 7, APC-Cy 7) Usually, as long as the antibody binds to the bead and is as bright or brighter than stained cells Should compensation controls be treated the same as experimental samples (e. g. , fixed and permeabilized)? n Yes, although with optimal fix/perm protocols this may make little difference Holden Maecker, Flow Cytometry Consulting

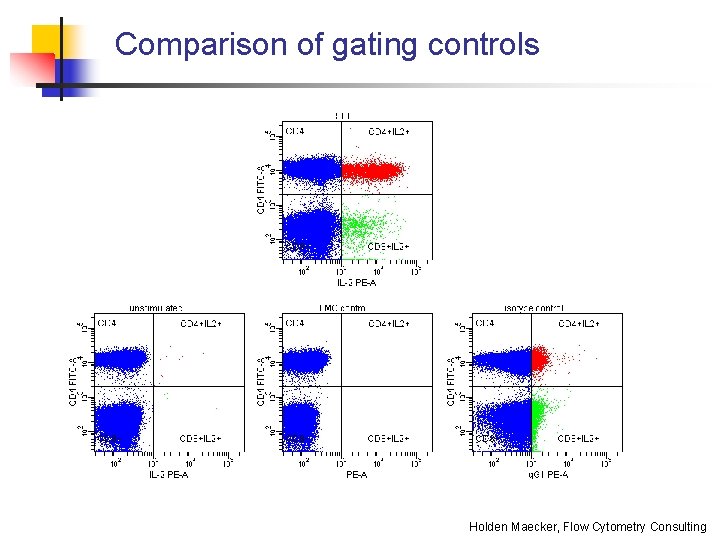
Comparison of gating controls Holden Maecker, Flow Cytometry Consulting

Consider using lyophilized reagents n n n Lyophilization provides increased stability, even at room temperature or 37 o. C One batch of reagents can be used for an entire longitudinal study Pre-configured plates can avoid errors of reagent addition Complex experiments (multiple stimuli, multiple polychromatic staining cocktails) become easier Lyophilized cell controls can provide run-to-run standardization Holden Maecker, Flow Cytometry Consulting

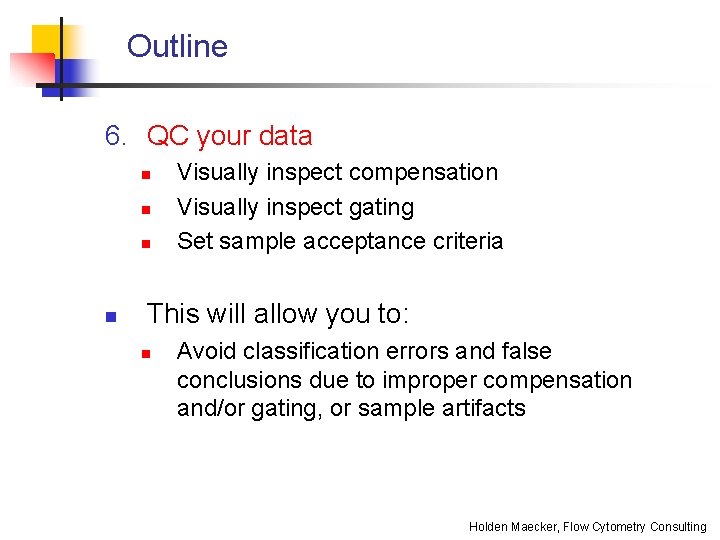
Outline 6. QC your data n n Visually inspect compensation Visually inspect gating Set sample acceptance criteria This will allow you to: n Avoid classification errors and false conclusions due to improper compensation and/or gating, or sample artifacts Holden Maecker, Flow Cytometry Consulting

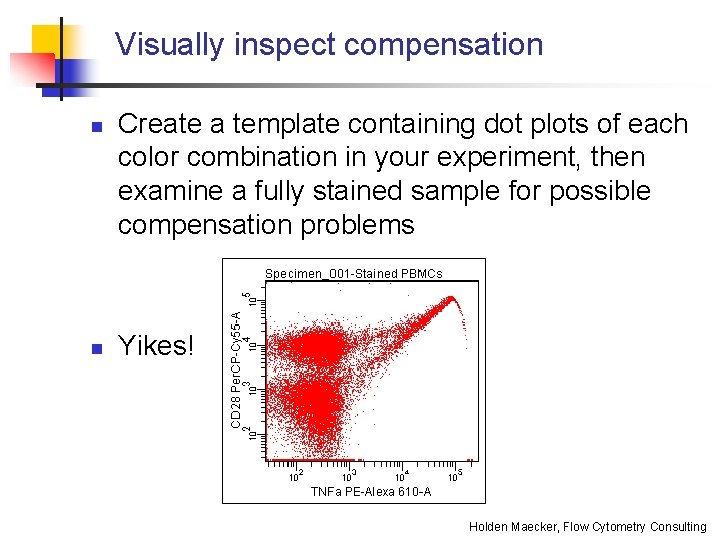
Visually inspect compensation n n Create a template containing dot plots of each color combination in your experiment, then examine a fully stained sample for possible compensation problems Yikes! Holden Maecker, Flow Cytometry Consulting

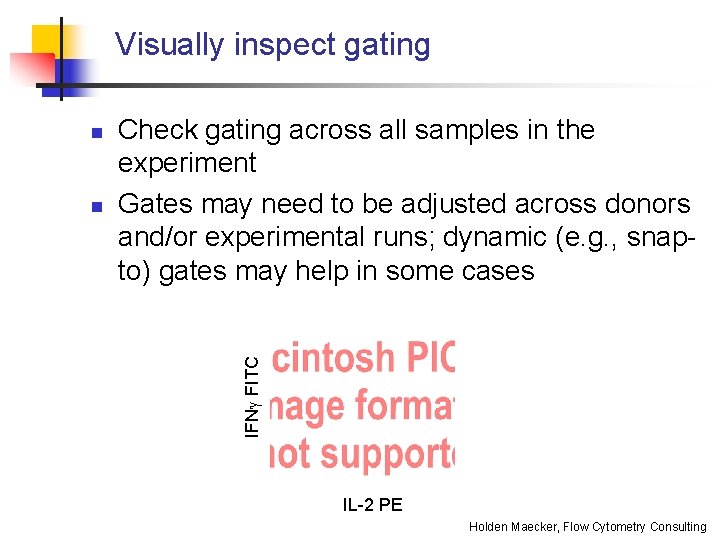
Visually inspect gating n Check gating across all samples in the experiment Gates may need to be adjusted across donors and/or experimental runs; dynamic (e. g. , snapto) gates may help in some cases IFNg FITC n IL-2 PE Holden Maecker, Flow Cytometry Consulting

Types of sample acceptance criteria n n n Minimum viability and recovery for cryopreserved PBMC Minimum number of events collected in an appropriate gate (e. g. , lymphocytes) Minimum number of events within a region of interest, to calculate an accurate percentage Holden Maecker, Flow Cytometry Consulting

Outline 1. Configure your instrument 2. Characterize your instrument 3. Design your panel 4. Optimize settings for your panel 5. Run appropriate controls 6. QC your data Holden Maecker, Flow Cytometry Consulting

A question for you to answer How many colors can you combine and still have robust results? This depends on: -The experimental question -The instrument used -The markers to be combined Holden Maecker, Flow Cytometry Consulting

References n n Maecker, H. T. , Frey, T. , Nomura, L. E. , and Trotter, J. 2004. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A 62: 169. Maecker, H. T. , and Trotter, J. 2006. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A 69: 1037. Roederer, M. 2008. How many events is enough? Are you positive? Cytometry A 73: 384 -385. Mc. Laughlin, B. E. , N. Baumgarth, M. Bigos, M. Roederer, S. C. De Rosa, J. D. Altman, D. F. Nixon, J. Ottinger, C. Oxford, T. G. Evans, and D. M. Asmuth. 2008. Nine-color flow cytometry for accurate measurement of T cell subsets and cytokine responses. Part I: Panel design by an empiric approach. Cytometry A 73: 400 -410. Holden Maecker, Flow Cytometry Consulting

Acknowledgements n n n n Laurel Nomura Margaret Inokuma Maria Suni Maria Jaimes Smita Ghanekar Jack Dunne Skip Maino § Joe Trotter § Dennis Sasaki § Marina Gever Holden Maecker, Flow Cytometry Consulting