States of matter Kinetic Theory All matter is









- Slides: 12

States of matter

Kinetic Theory • All matter is made up of tiny particles that are constantly moving

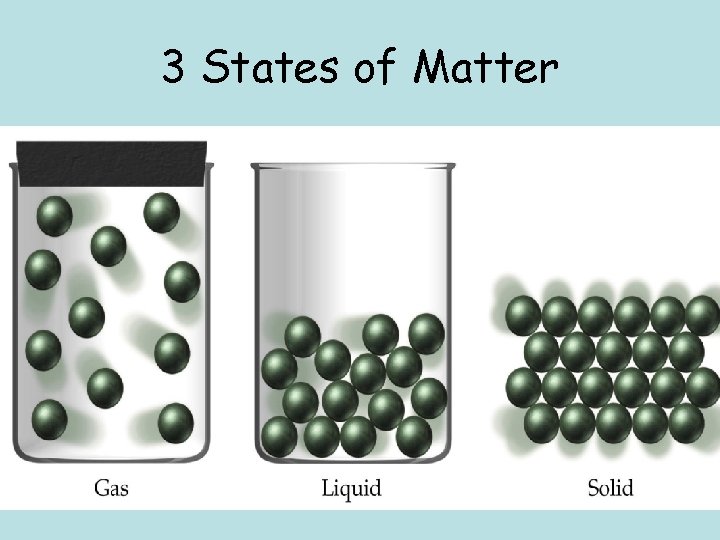
Solids • Have definite shape and definite volume • Particles are closely packed, vibrate in place, & held together by strong force of attraction

2 Types of solids 1. Crystalline solids: particles arranged in a regular repeating 3 D pattern Ex: salt, ice, diamonds

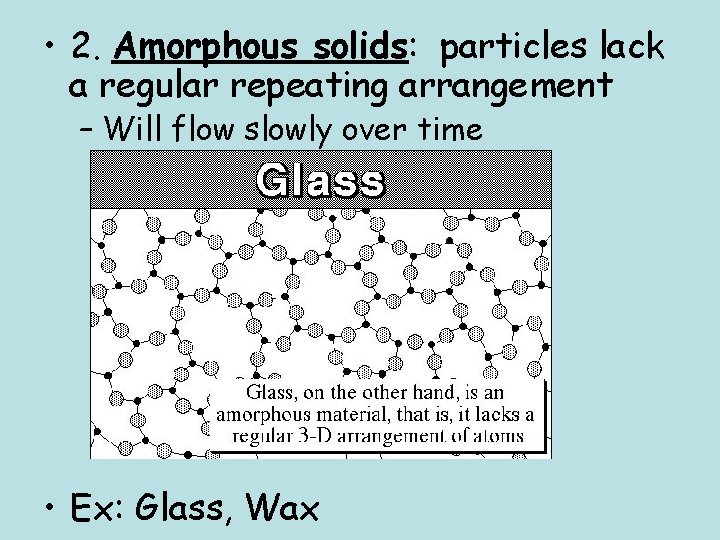
• 2. Amorphous solids: particles lack a regular repeating arrangement – Will flow slowly over time • Ex: Glass, Wax

Liquids • Have definite volume, not definite shape – Force of attraction not strong enough to hold a definite shape • Particles close together but move more than solids • Liquids flow and take shape of container

Liquids flow to take shape of their container

Gases • Have no definite shape and no definite volume • Particles move quickly and collide often • Particles very far apart • Force of attraction between particles is very weak • Gases expand will fill entire container

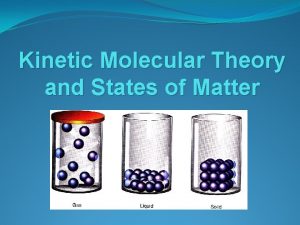
3 States of Matter

Plasma • 4 th state of matter (similar to gas) • No definite volume or shape • Most common form of matter in universe – Found in sun and stars • Particles collide violently at high temperatures – Collisions cause particles to become electrically charged


Changing States of Matter • Adding energy causes particles to move faster and further apart – Particles overcome forces of attraction allowing matter to change states (solid-liquid-gas)
 The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Explain, using kinetic particle theory, why gases diffuse.
Explain, using kinetic particle theory, why gases diffuse. What is a kinetic theory of matter
What is a kinetic theory of matter Four phase changes
Four phase changes John donne born
John donne born Name three line segments
Name three line segments There were 11
There were 11 Southern states
Southern states Checks and balances dbq
Checks and balances dbq Kinetic molecular theory of solid
Kinetic molecular theory of solid