SOURCES OF HEAT A NATURAL SOURCES 1 The






- Slides: 13


SOURCES OF HEAT A. NATURAL SOURCES 1. The sun 2. The interior of the Earth B. ARTIFICIAL SOURCES 1. Chemical Action 2. Mechanical Energy 3. Electrical Energy 4. Nuclear Energy

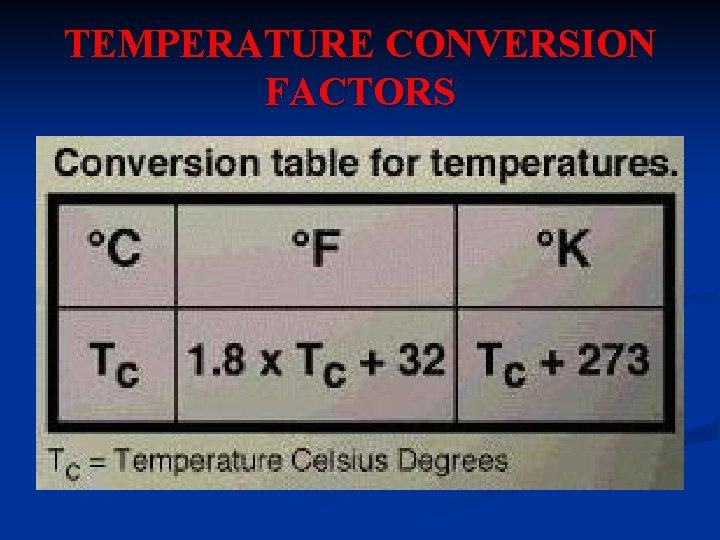
UNITS FOR TEMPERATURE 1. Fahrenheit Scale (o. F) – Fahrenheit temperature scale is a scale based on 32 for the freezing point of water and 212 for the boiling point of water, the interval between the two being divided into 180 parts. The 18 th-century German physicist Daniel Gabriel Fahrenheit originally took as the zero of his scale the temperature of an equal ice-salt mixture and selected the value of 98. 6 for normal body temperature.

2. Celsius Scale (o. C) - is based on 0 for the freezing point of water and 100 for the boiling point of water. Invented in 1742 by the Swedish astronomer Anders Celsius, it is sometimes called the centigrade scale because of the 100 -degree interval between the defined points.

3. Kelvin (K) - Kelvin temperature scale is the base unit of thermodynamic temperature measurement in the International System (SI) of measurement. It is defined as 1/ 273. 16 of the triple point (equilibrium among the solid, liquid, and gaseous phases) of pure water. Zero point absolute zero, theoretical temperature at which the molecules of a substance have the lowest energy. The kelvin has the same magnitude as the degree Celsius.

TEMPERATURE CONVERSION FACTORS


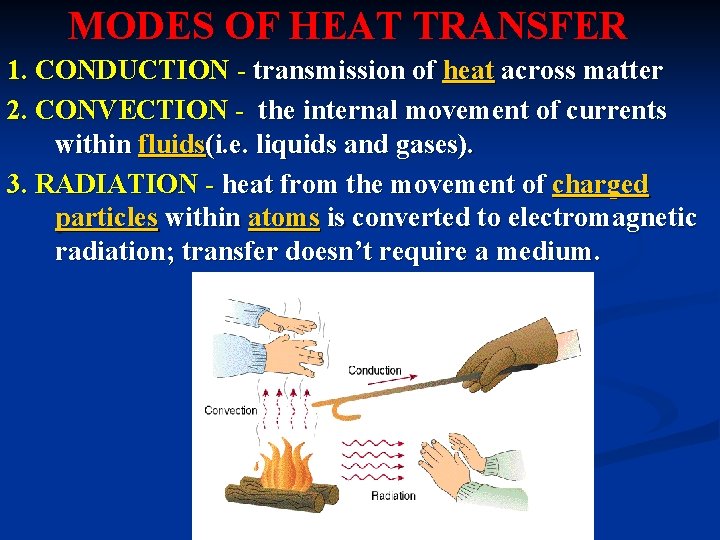
MODES OF HEAT TRANSFER 1. CONDUCTION - transmission of heat across matter 2. CONVECTION - the internal movement of currents within fluids(i. e. liquids and gases). 3. RADIATION - heat from the movement of charged particles within atoms is converted to electromagnetic radiation; transfer doesn’t require a medium.

EFFECTS OF HEAT 1. 2. 3. 4. 5. Change in temperature Change in phase Change in size Chemical change Change in bodily functions of living organisms

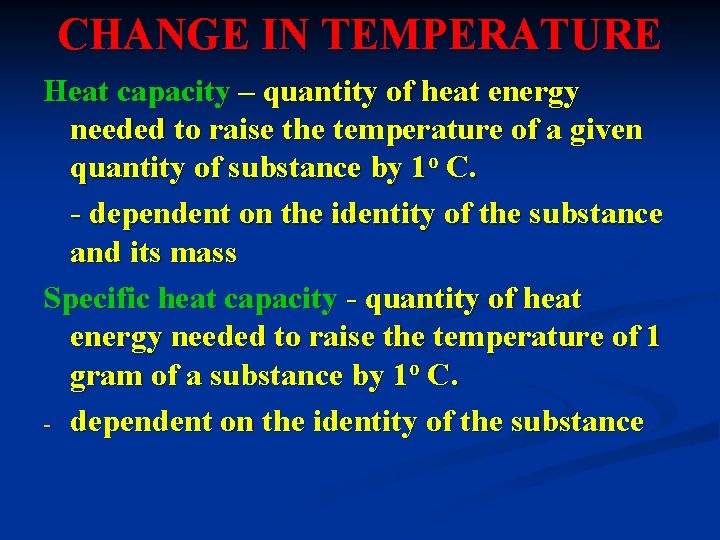
CHANGE IN TEMPERATURE Heat capacity – quantity of heat energy needed to raise the temperature of a given quantity of substance by 1 o C. - dependent on the identity of the substance and its mass Specific heat capacity - quantity of heat energy needed to raise the temperature of 1 gram of a substance by 1 o C. - dependent on the identity of the substance

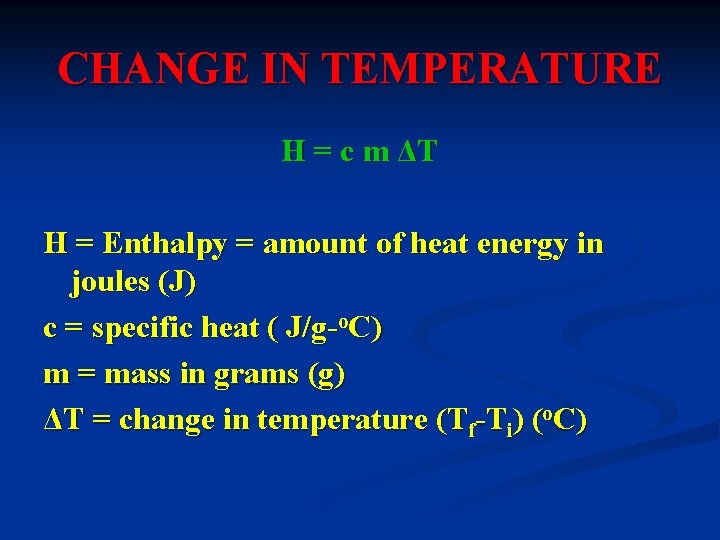
CHANGE IN TEMPERATURE H = c m ΔT H = Enthalpy = amount of heat energy in joules (J) c = specific heat ( J/g-o. C) m = mass in grams (g) ΔT = change in temperature (Tf-Ti) (o. C)

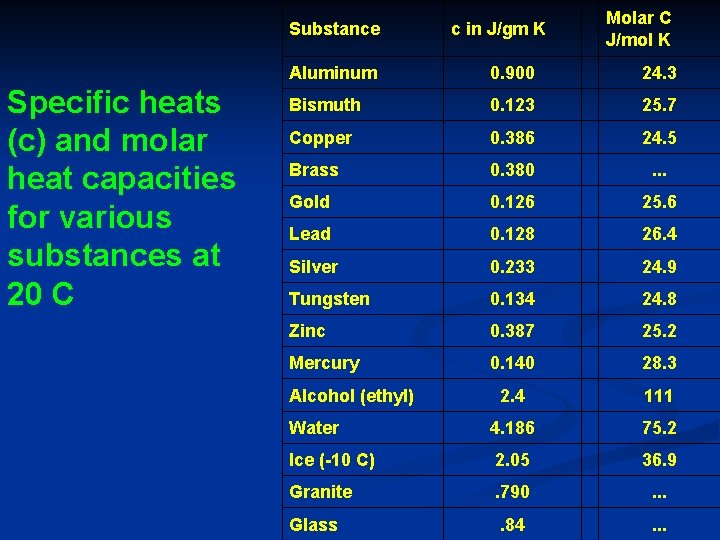
Substance Specific heats (c) and molar heat capacities for various substances at 20 C c in J/gm K Molar C J/mol K Aluminum 0. 900 24. 3 Bismuth 0. 123 25. 7 Copper 0. 386 24. 5 Brass 0. 380 . . . Gold 0. 126 25. 6 Lead 0. 128 26. 4 Silver 0. 233 24. 9 Tungsten 0. 134 24. 8 Zinc 0. 387 25. 2 Mercury 0. 140 28. 3 2. 4 111 Water 4. 186 75. 2 Ice (-10 C) 2. 05 36. 9 Granite . 790 . . . Glass . 84 . . . Alcohol (ethyl)

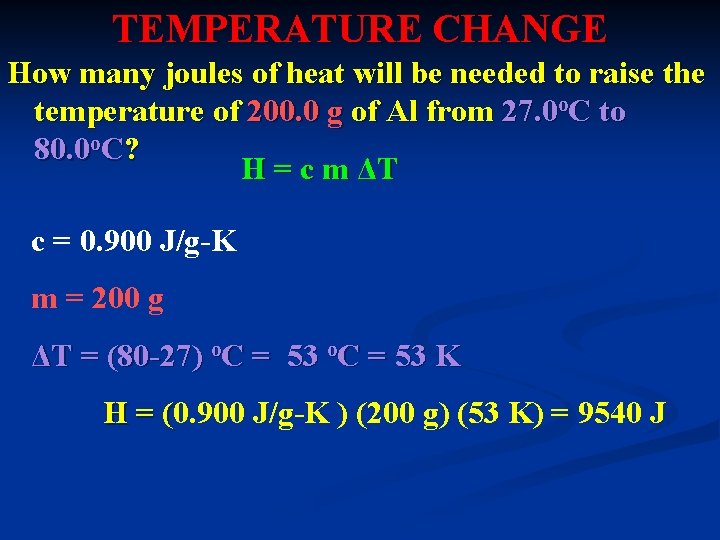
TEMPERATURE CHANGE How many joules of heat will be needed to raise the temperature of 200. 0 g of Al from 27. 0 o. C to 80. 0 o. C? H = c m ΔT c = 0. 900 J/g-K m = 200 g ΔT = (80 -27) o. C = 53 K H = (0. 900 J/g-K ) (200 g) (53 K) = 9540 J (
 Natural source of heat energy
Natural source of heat energy Source of heat
Source of heat Print sources of information
Print sources of information Importance water resources
Importance water resources Sources of heat
Sources of heat Organic nitrogen sources
Organic nitrogen sources Fluoride natural sources
Fluoride natural sources Nuclear energy facts
Nuclear energy facts Emulsifying agent in pharmacy
Emulsifying agent in pharmacy Natural sources of carbon monoxide
Natural sources of carbon monoxide Combination cooking method definition
Combination cooking method definition Spcific heat of water
Spcific heat of water Equation for specific latent heat of fusion
Equation for specific latent heat of fusion Natural hazards vs natural disasters
Natural hazards vs natural disasters