Slow Photoelectron Velocitymap Imaging Spectroscopy Etienne Garand Jia


- Slides: 17

Slow Photoelectron Velocity-map Imaging Spectroscopy Etienne Garand, Jia Zhou and Daniel M. Neumark Department of Chemistry, UC Berkeley, Berkeley CA 94720

Study of energetic neutral molecules n Radicals q Hydrocarbon combustion q Interstellar clouds q Upper atmosphere n Small clusters Transition state and pre-reactive complexes n How can we get information on the geometry and electronic structure of those molecules?

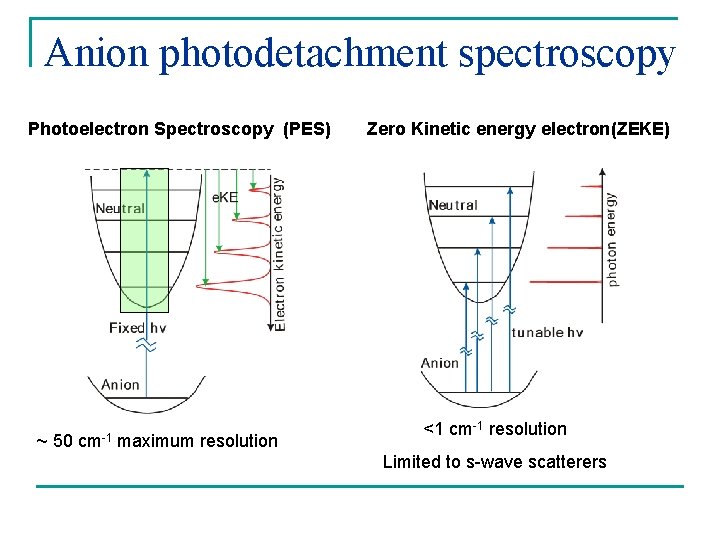
Anion photodetachment spectroscopy Photoelectron Spectroscopy (PES) ~ 50 cm-1 maximum resolution Zero Kinetic energy electron(ZEKE) <1 cm-1 resolution Limited to s-wave scatterers

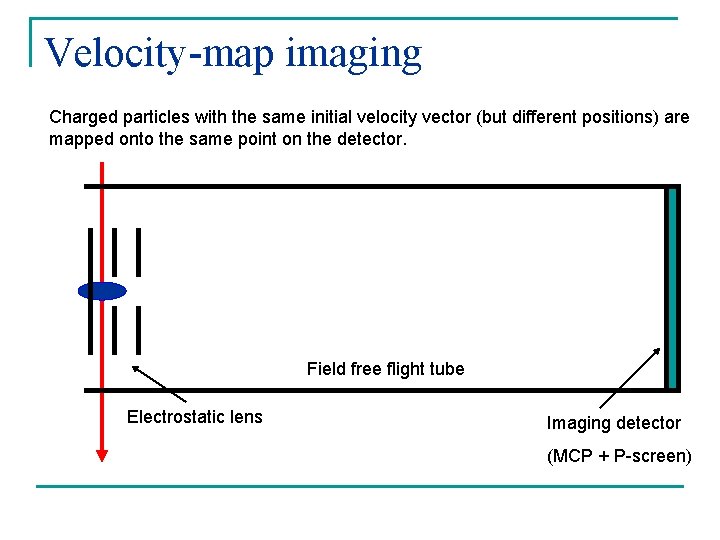
Velocity-map imaging Charged particles with the same initial velocity vector (but different positions) are mapped onto the same point on the detector. Field free flight tube Electrostatic lens Imaging detector (MCP + P-screen)

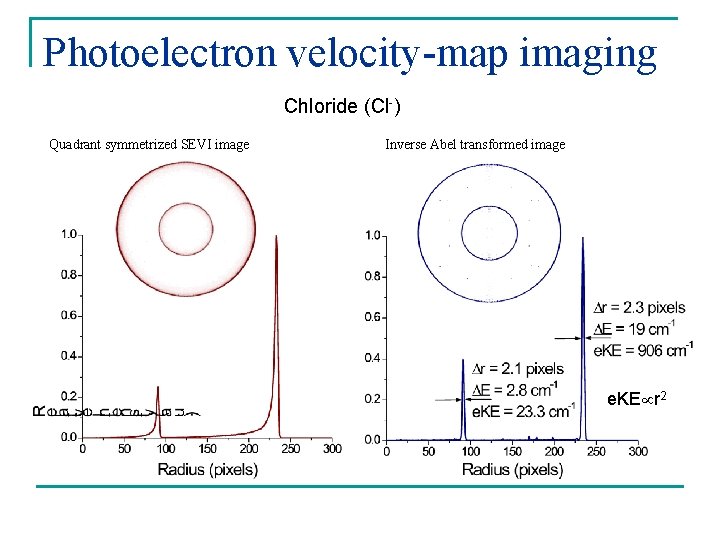
Photoelectron velocity-map imaging Chloride (Cl-) Quadrant symmetrized SEVI image Inverse Abel transformed image e. KE r 2

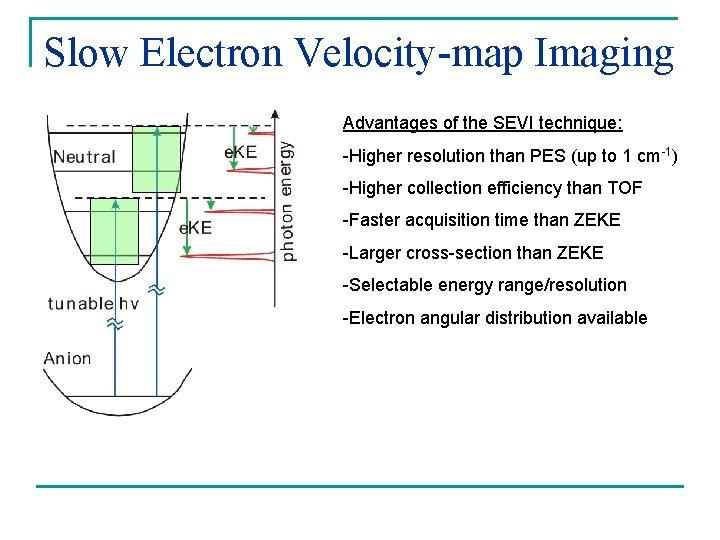
Slow Electron Velocity-map Imaging Advantages of the SEVI technique: -Higher resolution than PES (up to 1 cm-1) -Higher collection efficiency than TOF -Faster acquisition time than ZEKE -Larger cross-section than ZEKE -Selectable energy range/resolution -Electron angular distribution available

Examples of SEVI: C 2 H and C 4 H Important in combustion chemistry, photolysis processes, and interstellar chemistry 1) C≡C-H: Prototype of complex vibronic coupling - 2 S+ ground state with low-lying 2 P excited state (~3600 cm-1) - Renner-Teller coupling on the 2 P state - Pseudo Jahn-Teller between the 2 S+ and 2 P(A’) for all bent geometries 2) C≡C-H: Very close lying states - 2 S+ and 2 P states within <500 cm-1

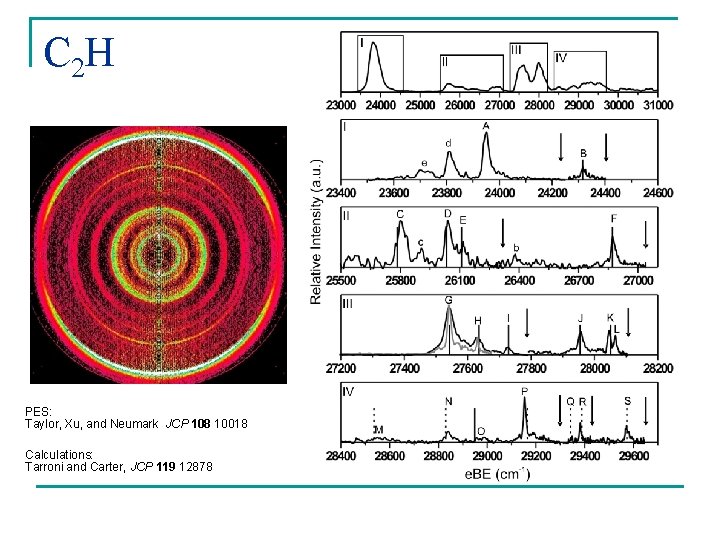
C 2 H PES: Taylor, Xu, and Neumark JCP 108 10018 Calculations: Tarroni and Carter, JCP 119 12878

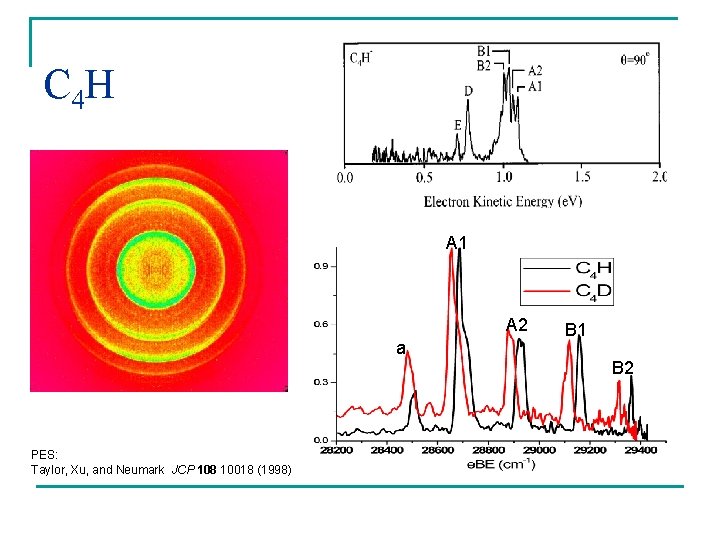
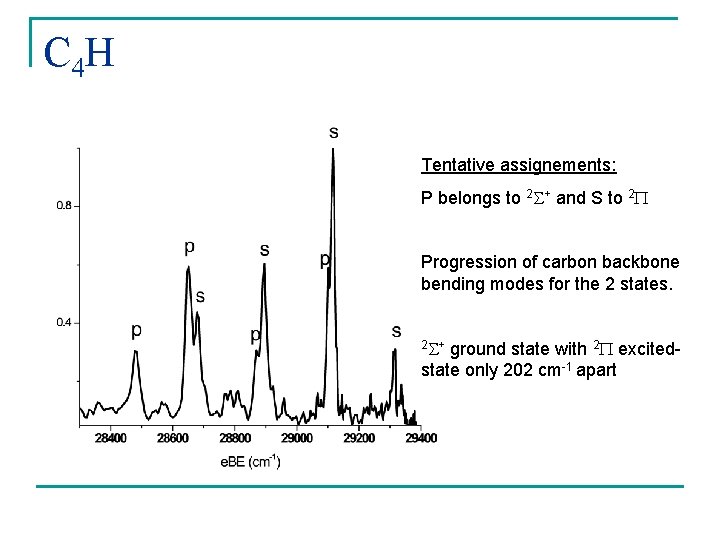
C 4 H A 1 A 2 a PES: Taylor, Xu, and Neumark JCP 108 10018 (1998) B 1 B 2

C 4 H Tentative assignements: P belongs to 2 S+ and S to 2 P Progression of carbon backbone bending modes for the 2 states. 2 S+ ground state with 2 P excitedstate only 202 cm-1 apart

Some take home messages n Resolution up to 1 cm-1 in atomic anions n Peak width limited by unresolved rotational profile in molecular systems n Several new transitions not resolved in the PES spectra are now observable n p-wave photodetachment observed very close to threshold (~200 cm-1). n Complete high-resolution spectra are obtained within few hours compared to several days for ZEKE.

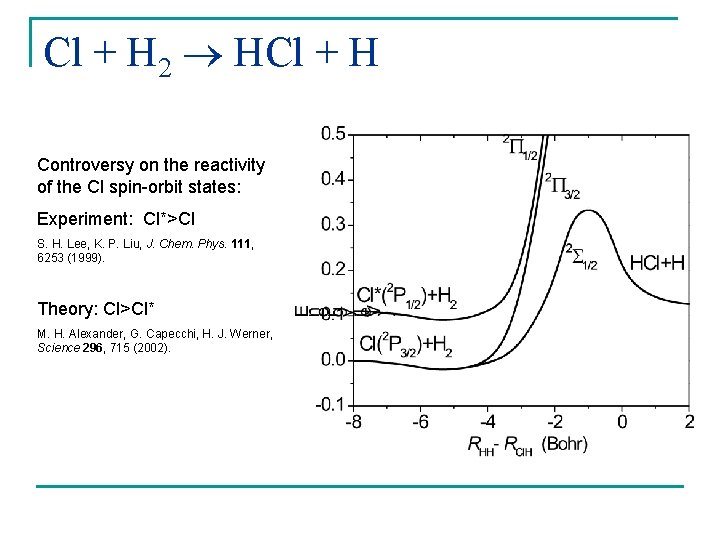
Cl + H 2 HCl + H Controversy on the reactivity of the Cl spin-orbit states: Experiment: Cl*>Cl S. H. Lee, K. P. Liu, J. Chem. Phys. 111, 6253 (1999). Theory: Cl>Cl* M. H. Alexander, G. Capecchi, H. J. Werner, Science 296, 715 (2002).

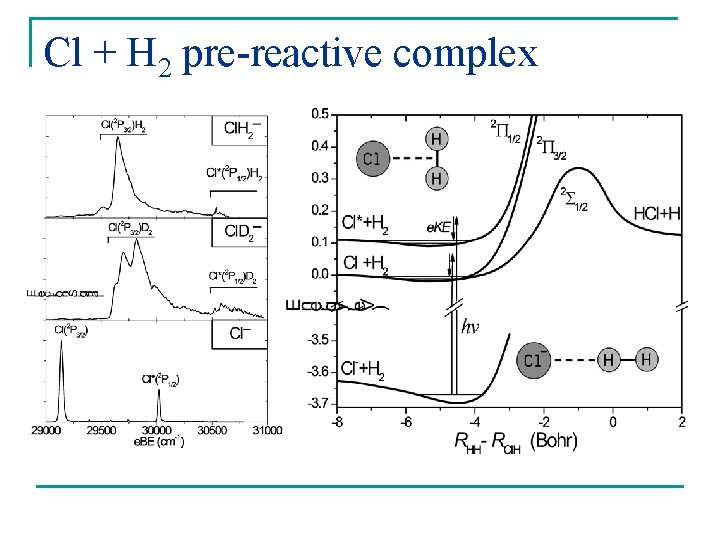
Cl + H 2 pre-reactive complex

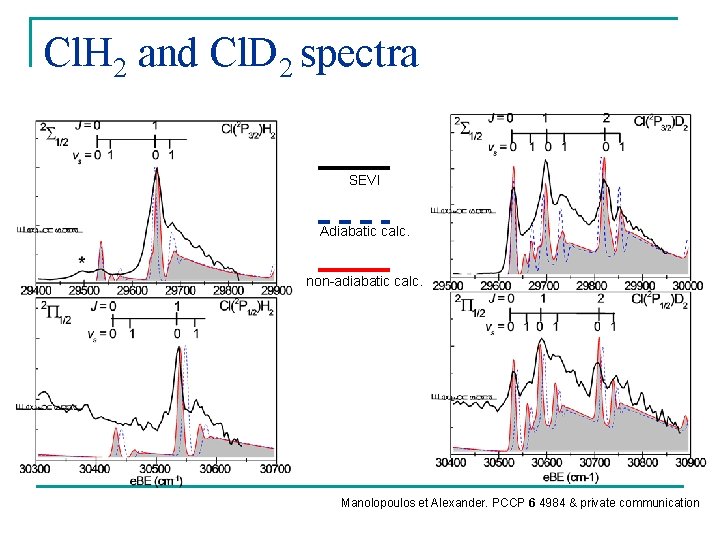
Cl. H 2 and Cl. D 2 spectra SEVI Adiabatic calc. non-adiabatic calc. Manolopoulos et Alexander. PCCP 6 4984 & private communication

Conclusions n Validate the accuracy of the Capecchi-Werner surfaces n Validate theoretical treatment of the couplings responsible for nonadiabatic coupling in the reactant valley. n Support the reactive scattering study of Alexander et al. n So what’s going on?

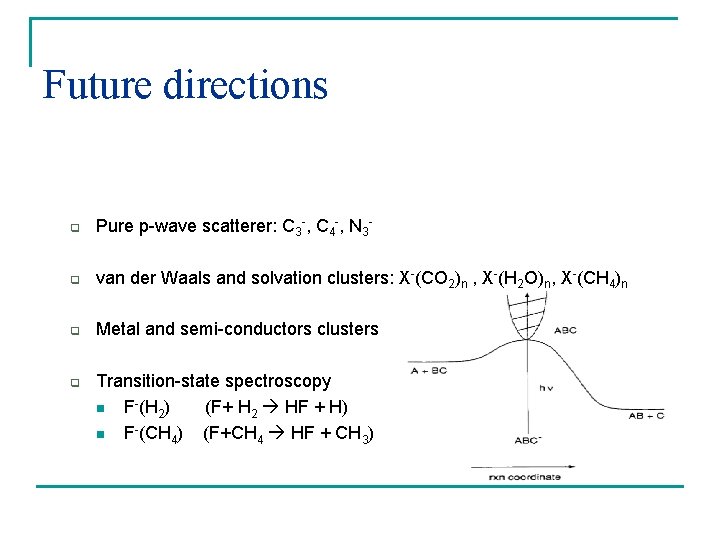
Future directions q Pure p-wave scatterer: C 3 -, C 4 -, N 3 - q van der Waals and solvation clusters: X-(CO 2)n , X-(H 2 O)n, X-(CH 4)n q Metal and semi-conductors clusters q Transition-state spectroscopy n F-(H 2) (F+ H 2 HF + H) n F-(CH 4) (F+CH 4 HF + CH 3)

Acknowledgements n Pr. Daniel M. Neumark n Jia Zhou (SEVI labmate) & Neumark’s group n Pr. M. H. Alexander and Pr. D. E Manolopoulos n Project funded by the AFOSR n Doctoral fellowship from the National Science and Engineering Research Council of Canada (NSERC)
 Xray photoelectron spectroscopy
Xray photoelectron spectroscopy Photoelectron spectroscopy
Photoelectron spectroscopy Spectroscopy ap chem
Spectroscopy ap chem Photoelectron spectrum of scandium
Photoelectron spectrum of scandium Smooth bore vs rifled
Smooth bore vs rifled Frc driver station mac
Frc driver station mac Copyright
Copyright Etienne ruppe
Etienne ruppe Etienne pfister
Etienne pfister Cicero translation theory
Cicero translation theory Realism
Realism Surcharge progressive
Surcharge progressive Susan mains artist
Susan mains artist Stiftung phönix zug
Stiftung phönix zug Etienne kairis
Etienne kairis Etienne bossut
Etienne bossut Dom perignon monk
Dom perignon monk Etienne forest
Etienne forest