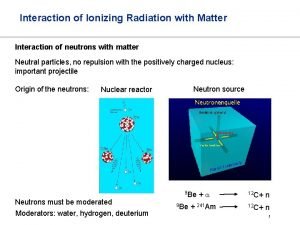
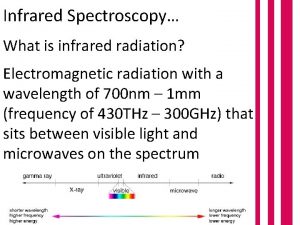
PHOTOELECTRON SPECTROSCOPY Spectroscopy uses interaction of electromagnetic radiation



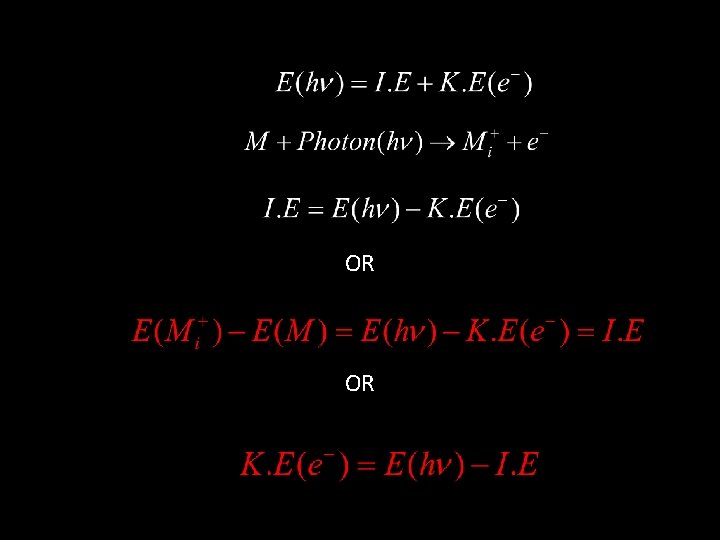
![[Co(NH 2 CH 2 NH 2)2(NO 2)2]NO 3 NH 2 CH 3 CH 2 [Co(NH 2 CH 2 NH 2)2(NO 2)2]NO 3 NH 2 CH 3 CH 2](https://slidetodoc.com/presentation_image/984e9f674ed7213a4fd881b1dd2bce64/image-24.jpg)



- Slides: 31

PHOTOELECTRON SPECTROSCOPY

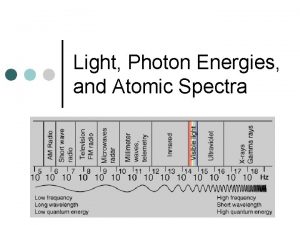
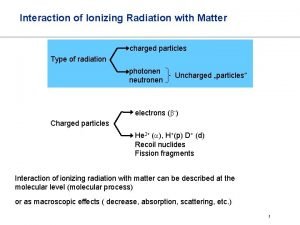
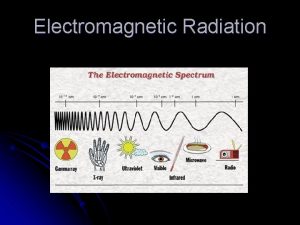
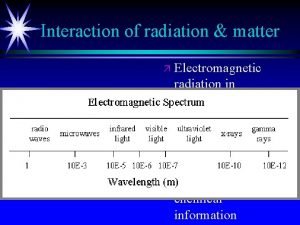
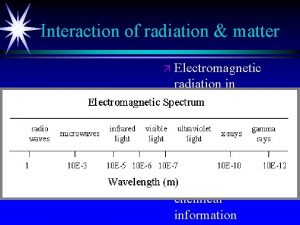
Spectroscopy uses interaction of electromagnetic radiation with matter to learn something about the matter. Characteristics of absorbed, emitted or scattered electromegnetic radiation are measured Photoelectron spectroscopy is entirely different! Kinetic energies of the ionized electrons are monitored It is an extension of PHOTOELECTRIC EFFECT

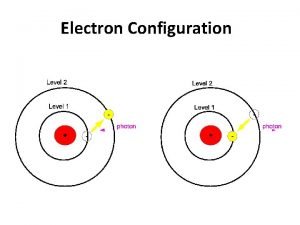
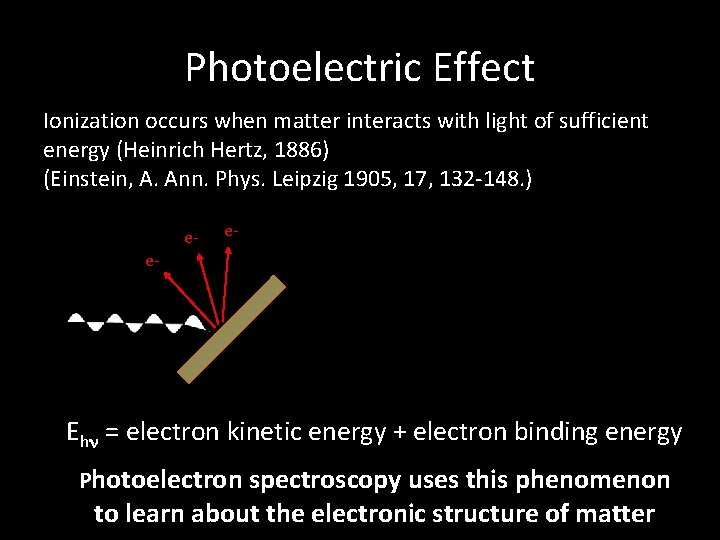
Photoelectric Effect Ionization occurs when matter interacts with light of sufficient energy (Heinrich Hertz, 1886) (Einstein, A. Ann. Phys. Leipzig 1905, 17, 132 -148. ) e- e- e- hn Ehn = electron kinetic energy + electron binding energy Photoelectron spectroscopy uses this phenomenon to learn about the electronic structure of matter

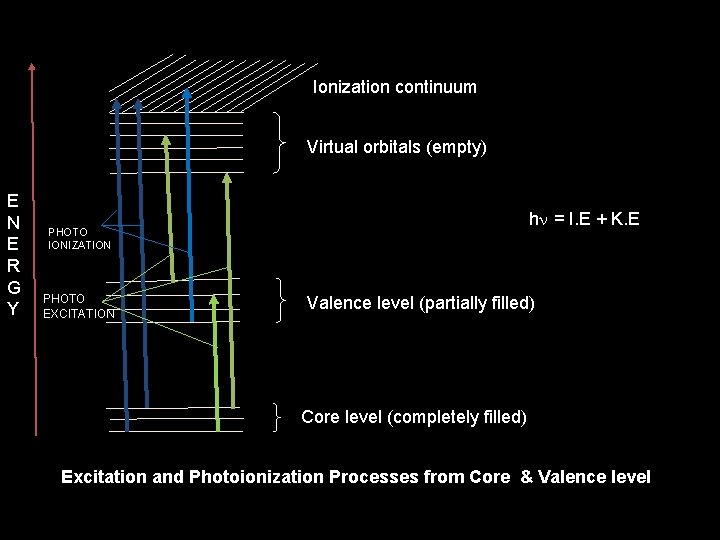
Ionization continuum Virtual orbitals (empty) E N E R G Y hn = I. E + K. E PHOTO IONIZATION PHOTO EXCITATION Valence level (partially filled) Core level (completely filled) Excitation and Photoionization Processes from Core & Valence level

Photoelectron spectroscopy is based on Einstein's photoelectric effect. A photon can ionize an electron from a molecule if the photon has an energy greater than the energy holding the electron in the molecule. Any photon energy in excess of that needed for ionization is carried by the outgoing electron in the form of kinetic energy.
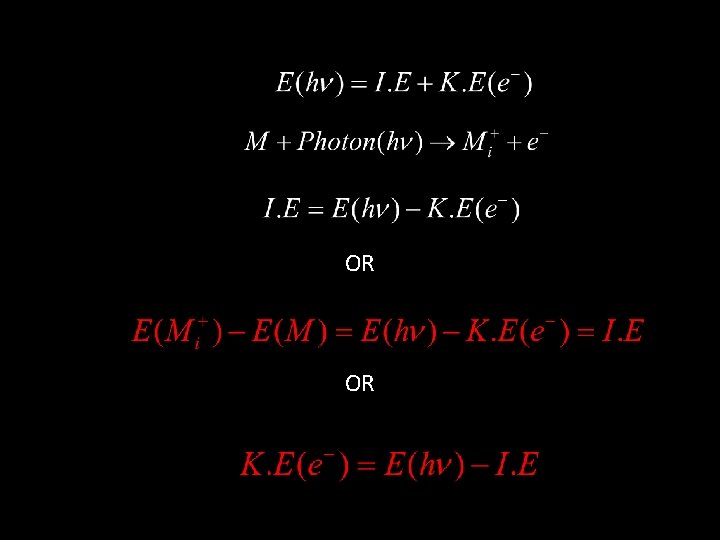
OR OR

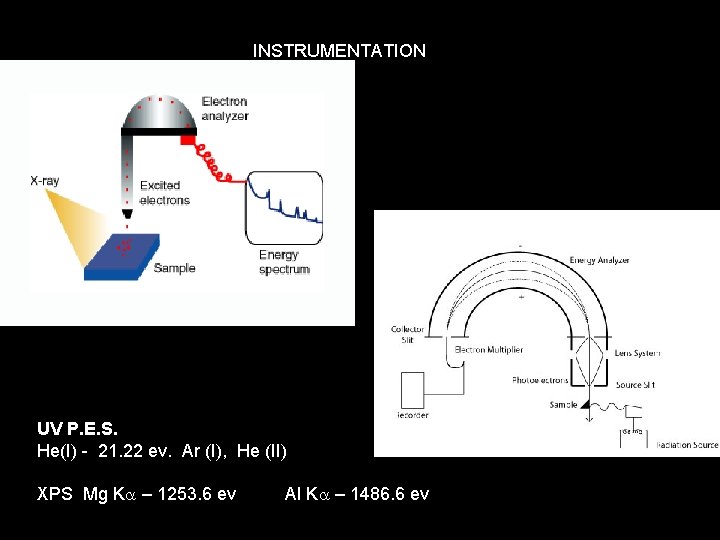
INSTRUMENTATION UV P. E. S. He(I) - 21. 22 ev. Ar (I), He (II) XPS Mg Ka – 1253. 6 ev Al Ka – 1486. 6 ev

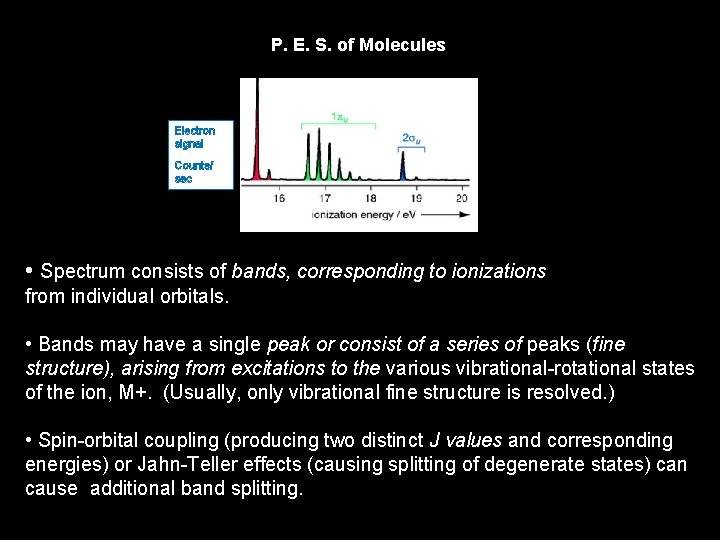
P. E. S. of Molecules Electron signal Counts/s secsecec • Spectrum consists of bands, corresponding to ionizations from individual orbitals. • Bands may have a single peak or consist of a series of peaks (fine structure), arising from excitations to the various vibrational-rotational states of the ion, M+. (Usually, only vibrational fine structure is resolved. ) • Spin-orbital coupling (producing two distinct J values and corresponding energies) or Jahn-Teller effects (causing splitting of degenerate states) can cause additional band splitting.

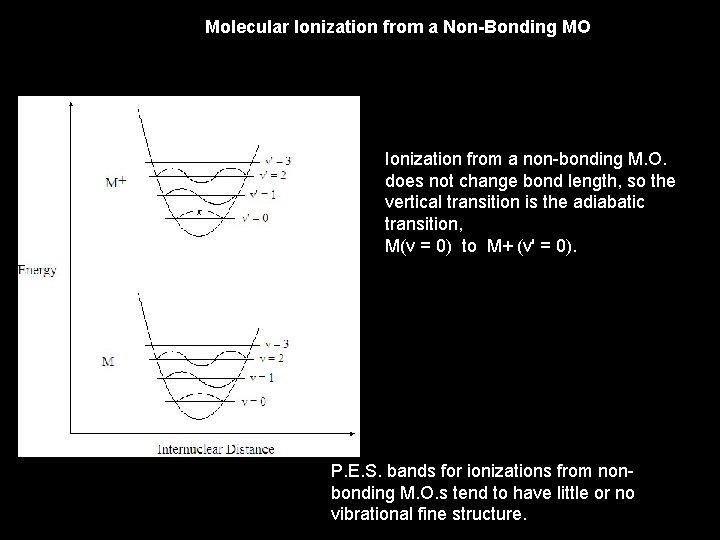
Molecular Ionization from a Non-Bonding MO Ionization from a non-bonding M. O. does not change bond length, so the vertical transition is the adiabatic transition, M(v = 0) to M+ (v' = 0). P. E. S. bands for ionizations from nonbonding M. O. s tend to have little or no vibrational fine structure.

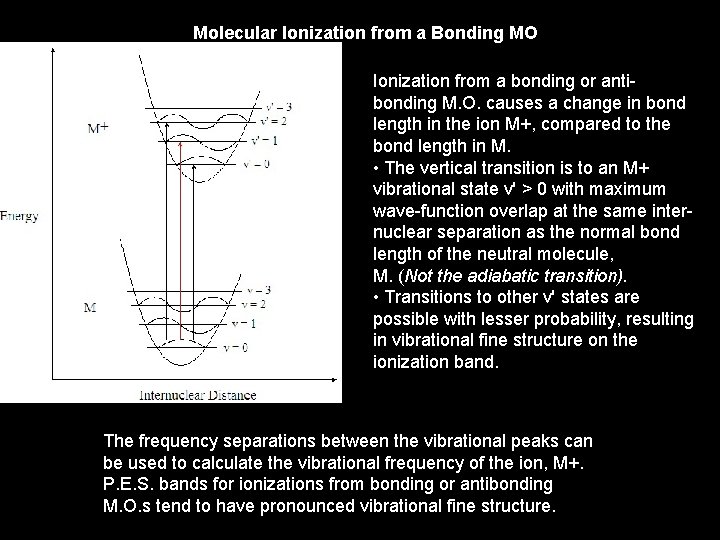
Molecular Ionization from a Bonding MO Ionization from a bonding or antibonding M. O. causes a change in bond length in the ion M+, compared to the bond length in M. • The vertical transition is to an M+ vibrational state v' > 0 with maximum wave-function overlap at the same internuclear separation as the normal bond length of the neutral molecule, M. (Not the adiabatic transition). • Transitions to other v' states are possible with lesser probability, resulting in vibrational fine structure on the ionization band. The frequency separations between the vibrational peaks can be used to calculate the vibrational frequency of the ion, M+. P. E. S. bands for ionizations from bonding or antibonding M. O. s tend to have pronounced vibrational fine structure.

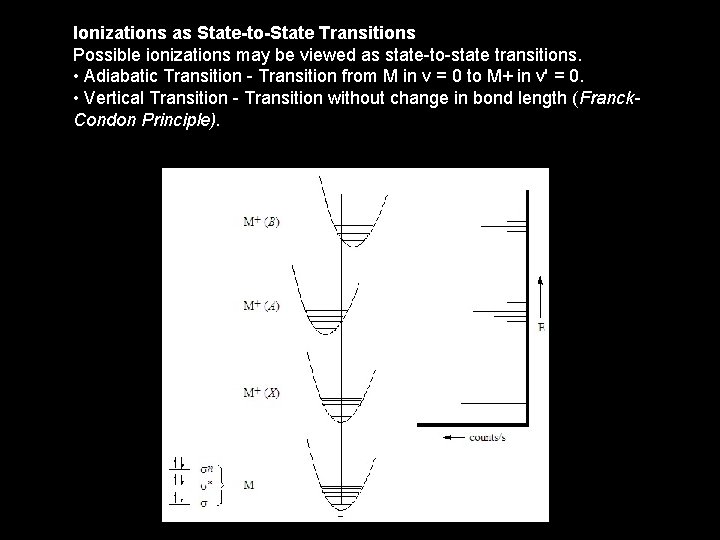
Ionizations as State-to-State Transitions Possible ionizations may be viewed as state-to-state transitions. • Adiabatic Transition - Transition from M in v = 0 to M+ in v' = 0. • Vertical Transition - Transition without change in bond length (Franck. Condon Principle).

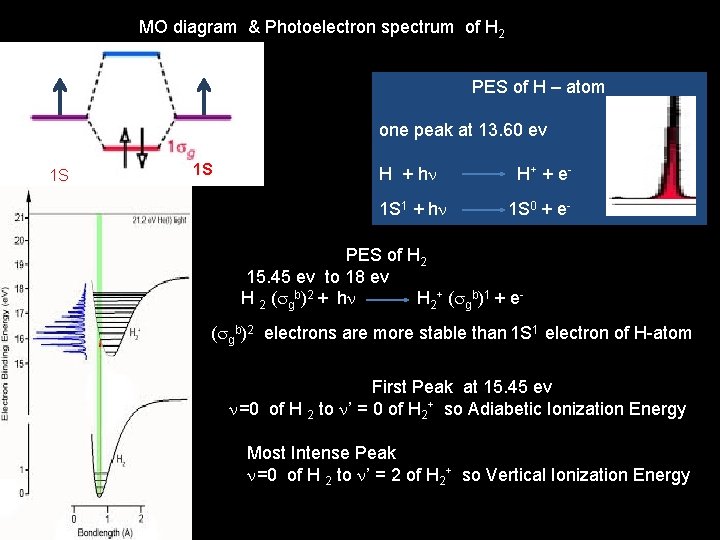
MO diagram & Photoelectron spectrum of H 2 PES of H – atom one peak at 13. 60 ev 1 S 1 S H + hn H+ + e 1 S 1 + hn 1 S 0 + e. PES of H 2 15. 45 ev to 18 ev H 2 (sgb)2 + hn H 2+ (sgb)1 + e- (sgb)2 electrons are more stable than 1 S 1 electron of H-atom First Peak at 15. 45 ev n=0 of H 2 to n’ = 0 of H 2+ so Adiabetic Ionization Energy Most Intense Peak n=0 of H 2 to n’ = 2 of H 2+ so Vertical Ionization Energy

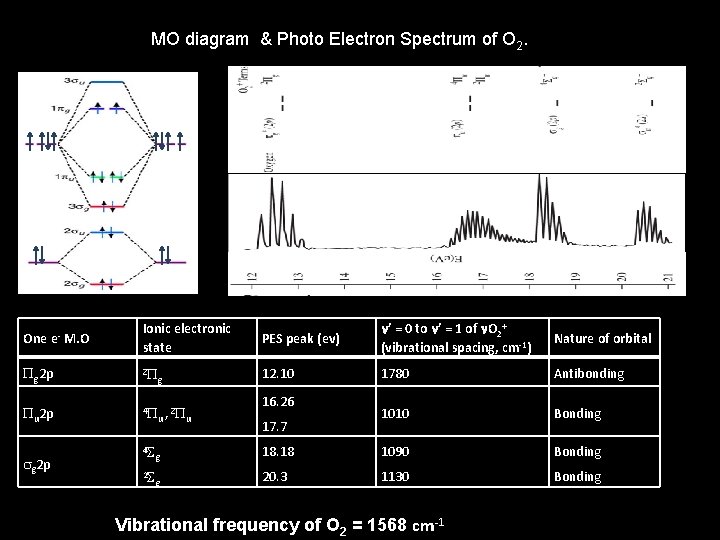
MO diagram & Photo Electron Spectrum of O 2. One e- M. O Ionic electronic state PES peak (ev) n’ = 0 to n’ = 1 of n. O 2+ (vibrational spacing, cm-1) Nature of orbital Pg 2 p 2 P g 12. 10 1780 Antibonding Pu 2 p 4 P 2 u , Pu 1010 Bonding sg 2 p 16. 26 17. 7 4 S g 18. 18 1090 Bonding 2 S g 20. 3 1130 Bonding Vibrational frequency of O 2 = 1568 cm-1

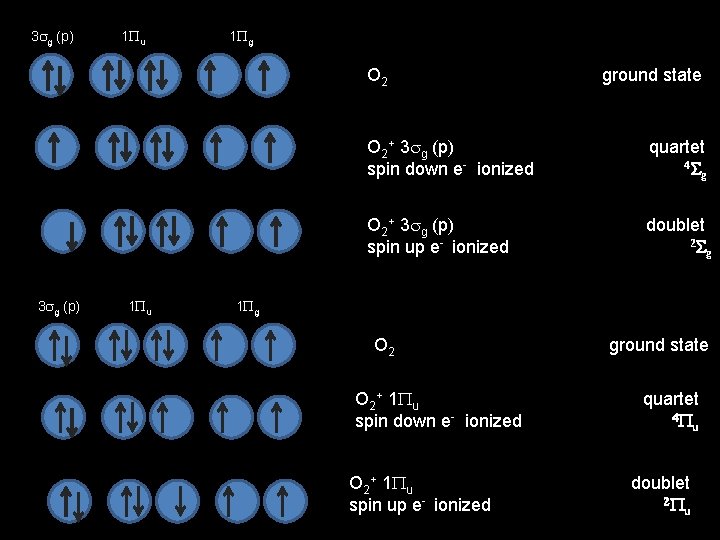
3 sg (p) 1 Pu 1 Pg O 2 ground state O 2+ 3 sg (p) quartet spin down e- ionized 4 Sg O 2+ 3 sg (p) doublet spin up e- ionized 2 Sg 3 sg (p) 1 Pu 1 Pg O 2 ground state O 2+ 1 Pu quartet spin down e- ionized 4 Pu O 2+ 1 Pu doublet spin up e- ionized 2 Pu

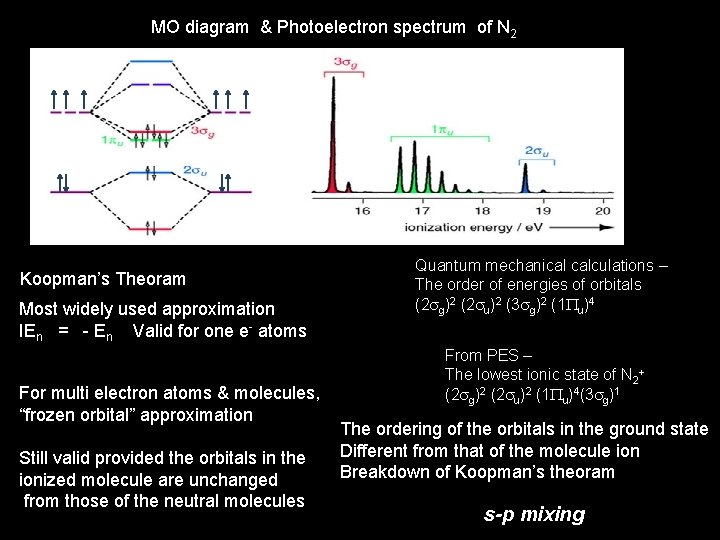
MO diagram & Photoelectron spectrum of N 2 Koopman’s Theoram Most widely used approximation IEn = - En Valid for one e- atoms For multi electron atoms & molecules, “frozen orbital” approximation Still valid provided the orbitals in the ionized molecule are unchanged from those of the neutral molecules Quantum mechanical calculations – The order of energies of orbitals (2 sg)2 (2 su)2 (3 sg)2 (1 Pu)4 From PES – The lowest ionic state of N 2+ (2 sg)2 (2 su)2 (1 Pu)4(3 sg)1 The ordering of the orbitals in the ground state Different from that of the molecule ion Breakdown of Koopman’s theoram s-p mixing

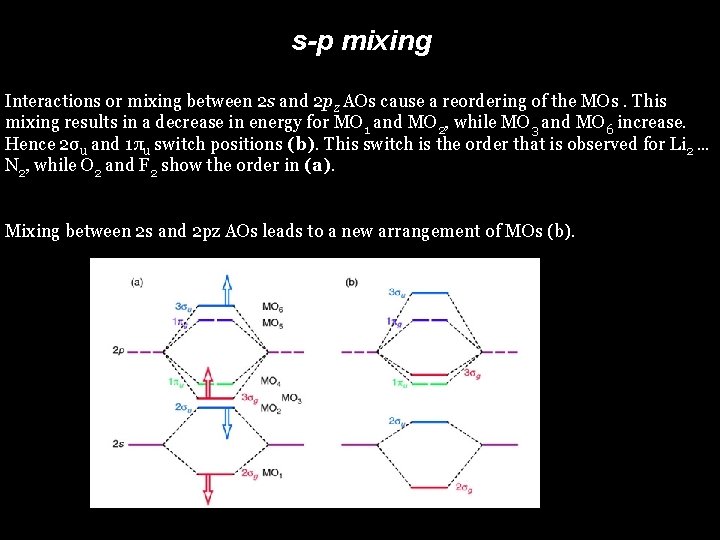
s-p mixing Interactions or mixing between 2 s and 2 pz AOs cause a reordering of the MOs. This mixing results in a decrease in energy for MO 1 and MO 2, while MO 3 and MO 6 increase. Hence 2σu and 1πu switch positions (b). This switch is the order that is observed for Li 2 … N 2, while O 2 and F 2 show the order in (a). Mixing between 2 s and 2 pz AOs leads to a new arrangement of MOs (b).

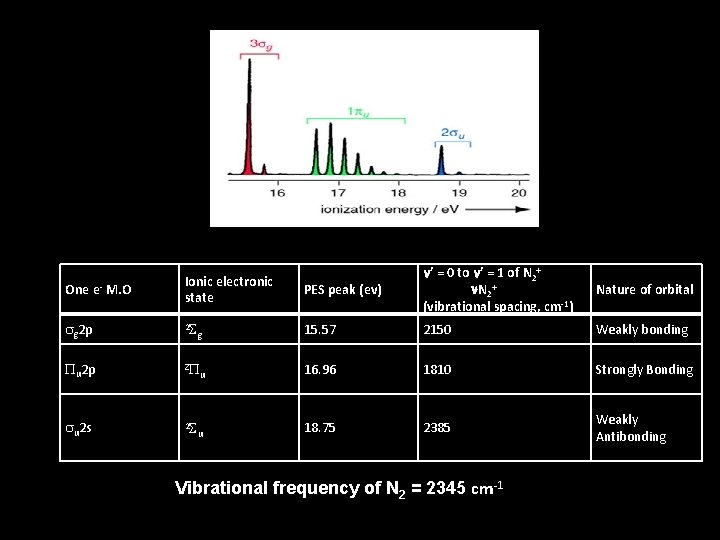
PES peak (ev) n’ = 0 to n’ = 1 of N 2+ n. N 2+ (vibrational spacing, cm-1) Nature of orbital g 15. 57 2150 Weakly bonding Pu 2 p 2 P u 16. 96 1810 Strongly Bonding su 2 s 2 S u 18. 75 2385 Weakly Antibonding One e- M. O Ionic electronic state sg 2 p 2 S Vibrational frequency of N 2 = 2345 cm-1

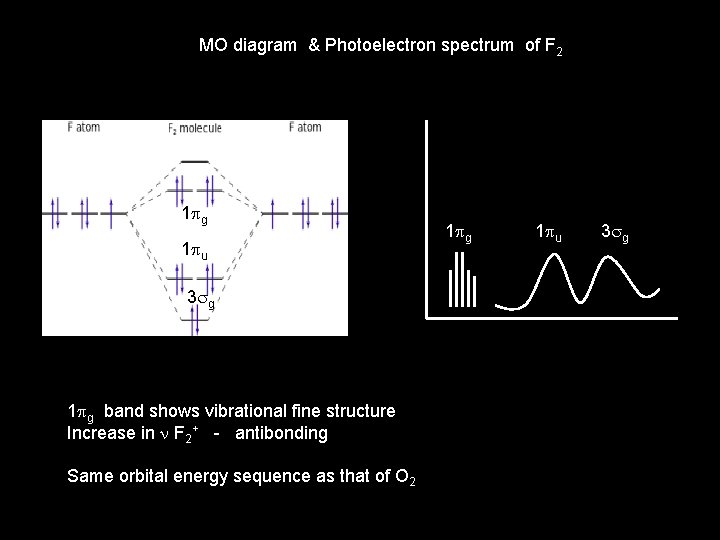
MO diagram & Photoelectron spectrum of F 2 1 pg 1 pu 3 sg 1 pg band shows vibrational fine structure Increase in n F 2+ - antibonding Same orbital energy sequence as that of O 2 1 pg 1 pu 3 sg

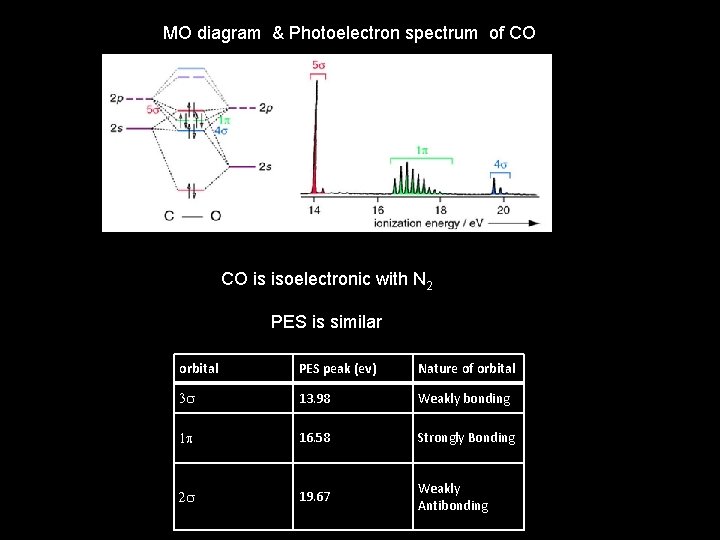
MO diagram & Photoelectron spectrum of CO CO is isoelectronic with N 2 PES is similar orbital PES peak (ev) Nature of orbital 3 s 13. 98 Weakly bonding 1 p 16. 58 Strongly Bonding 2 s 19. 67 Weakly Antibonding

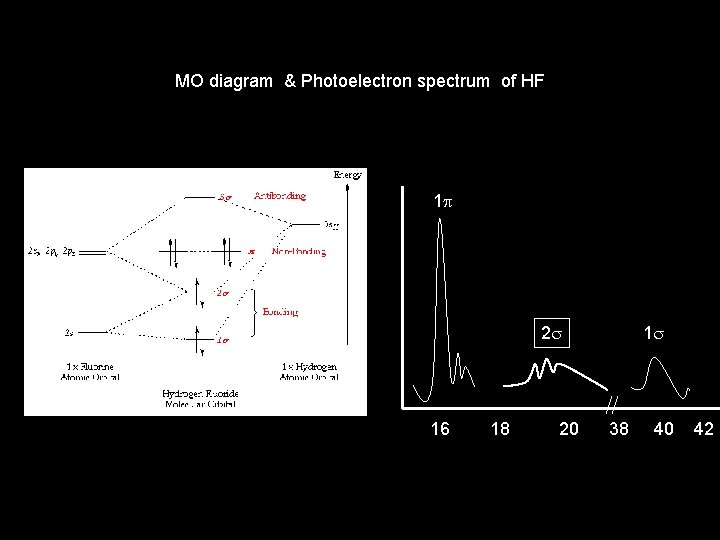
MO diagram & Photoelectron spectrum of HF 1 p 2 s 1 s 16 18 20 38 40 42

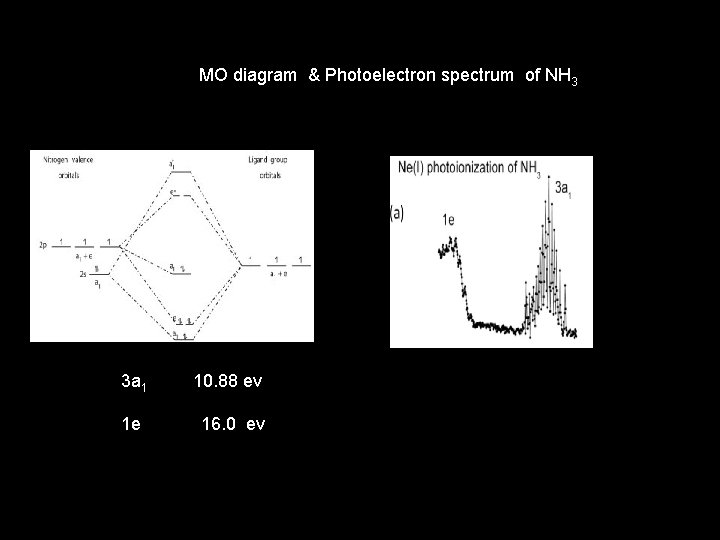
MO diagram & Photoelectron spectrum of NH 3 3 a 1 10. 88 ev 1 e 16. 0 ev

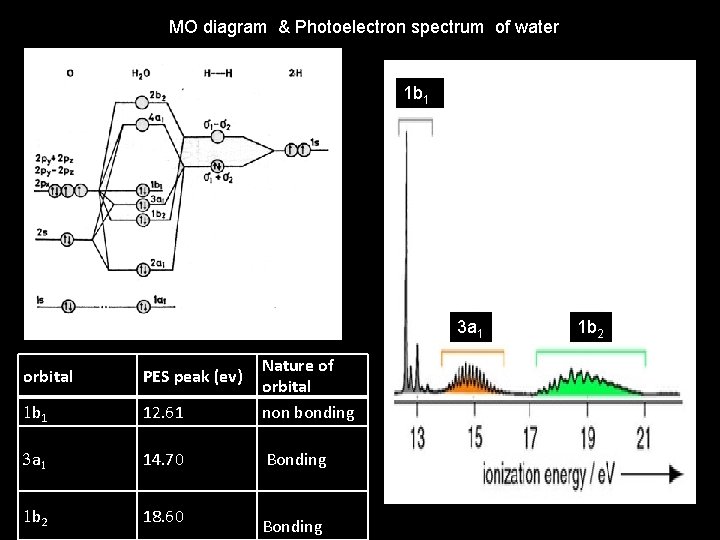
MO diagram & Photoelectron spectrum of water 1 b 1 3 a 1 orbital PES peak (ev) 1 b 1 12. 61 Nature of orbital non bonding 3 a 1 14. 70 Bonding 1 b 2 18. 60 Bonding 1 b 2

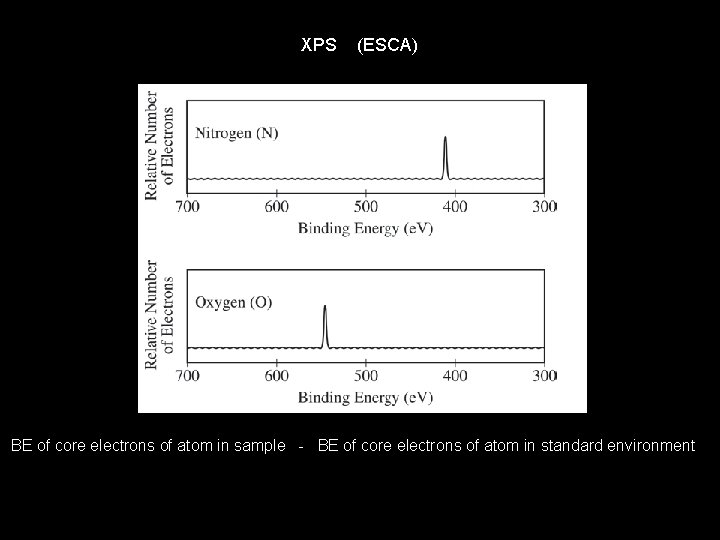
XPS (ESCA) BE of core electrons of atom in sample - BE of core electrons of atom in standard environment
![CoNH 2 CH 2 NH 22NO 22NO 3 NH 2 CH 3 CH 2 [Co(NH 2 CH 2 NH 2)2(NO 2)2]NO 3 NH 2 CH 3 CH 2](https://slidetodoc.com/presentation_image/984e9f674ed7213a4fd881b1dd2bce64/image-24.jpg)
[Co(NH 2 CH 2 NH 2)2(NO 2)2]NO 3 NH 2 CH 3 CH 2 COONa 1: 3 4: 2: 1 NO 2 NO 3 CH 3 CH 2 COONa 1: 2 CH 3 COONa 1: 1 HCOONa C 1 S XPS CF 3 COOCH 2 CH 3 IP CO : CH 1: 0 280 285 290 IP

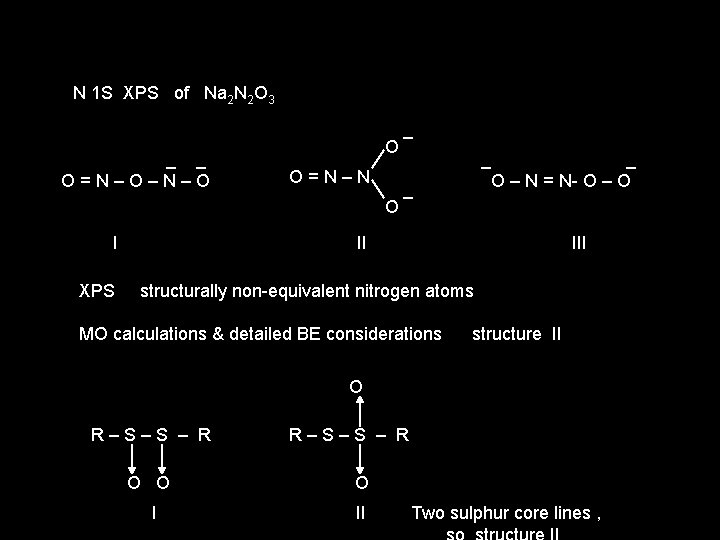
N 1 S XPS of Na 2 N 2 O 3 _ _ O = N – O – N – O O O = N – N O _ _ _ _ O – N = N- O – O I II III XPS structurally non-equivalent nitrogen atoms MO calculations & detailed BE considerations structure II O R – S – S – R O I II Two sulphur core lines ,

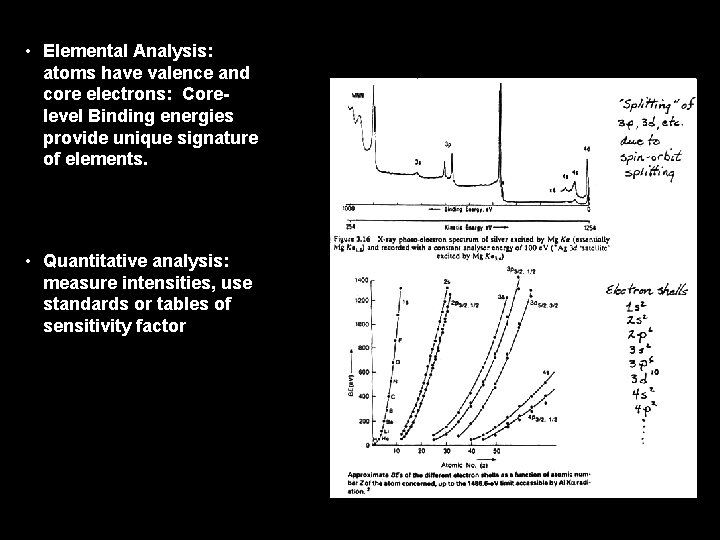
• Elemental Analysis: atoms have valence and core electrons: Corelevel Binding energies provide unique signature of elements. • Quantitative analysis: measure intensities, use standards or tables of sensitivity factor

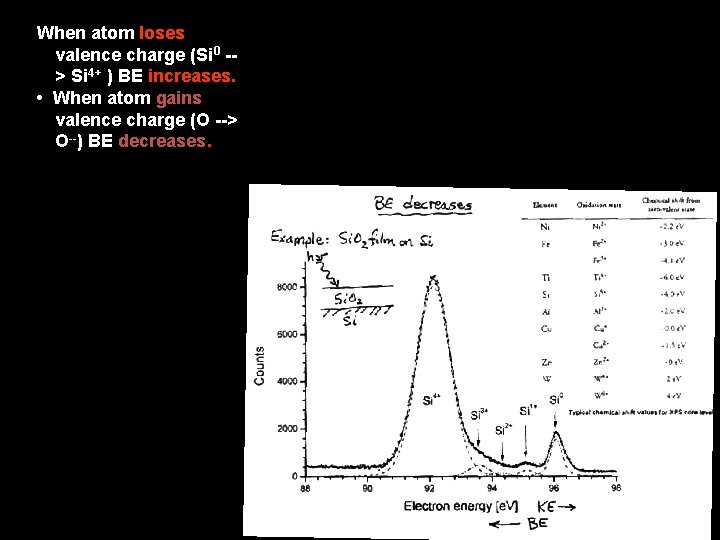
When atom loses valence charge (Si 0 -> Si 4+ ) BE increases. • When atom gains valence charge (O --> O--) BE decreases.

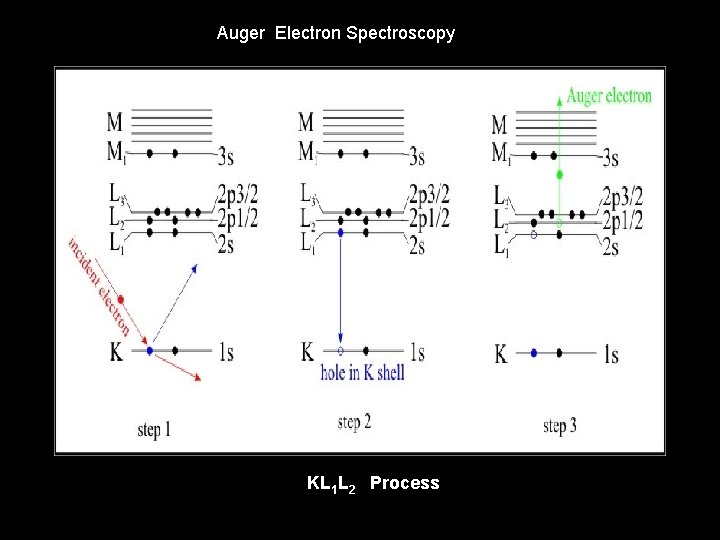
Auger Electron Spectroscopy KL 1 L 2 Process

Summary • PES is a fairly new technique, continuing to develop • PES has unique features compared to other spectroscopies • Valence spectroscopy: information on bonding • Core spectroscopy: qualitative and quantitative analysis, “chemical shift”

References International series of Monographs, Vol. 53: Photoelectron Spectroscopy, D. Beckerand D. Betteridge Physical Methods for Chemists, Russell S. Drago Electronic Absorption Spectroscopy and Related Techniques D. N. Satyanarayana

THANK YOU
 Photoelectron spectroscopy pogil
Photoelectron spectroscopy pogil Magnesium pes spectrum
Magnesium pes spectrum Xray photoelectron spectroscopy
Xray photoelectron spectroscopy Draw a photoelectron spectrum for aluminum
Draw a photoelectron spectrum for aluminum Spectrums of light
Spectrums of light Intensity of em waves
Intensity of em waves Intensity of electromagnetic radiation
Intensity of electromagnetic radiation When electromagnetic radiation of wavelength 300
When electromagnetic radiation of wavelength 300 Facts about electromagnetic radiation
Facts about electromagnetic radiation Which telescope detects invisible electromagnetic radiation
Which telescope detects invisible electromagnetic radiation Em waves
Em waves Wavelength formulas
Wavelength formulas Electromagnetic waves are longitudinal waves true or false
Electromagnetic waves are longitudinal waves true or false Types of radiation in the electromagnetic spectrum
Types of radiation in the electromagnetic spectrum Wkipedia
Wkipedia What is interaction of radiation with matter
What is interaction of radiation with matter Principal of mass spectroscopy
Principal of mass spectroscopy Microwave uses radiation
Microwave uses radiation Electric generator electromagnetic induction
Electric generator electromagnetic induction What are the 4 universal forces?
What are the 4 universal forces? Electromagnetic spectrum micrometers
Electromagnetic spectrum micrometers Electromagnetic induction
Electromagnetic induction Mechanical waves and electromagnetic waves similarities
Mechanical waves and electromagnetic waves similarities Conclusion of electromagnetic induction
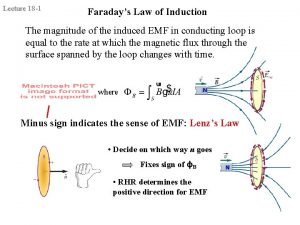
Conclusion of electromagnetic induction Faradays law
Faradays law Energy density in electromagnetic waves
Energy density in electromagnetic waves Electromagnetic spectrum
Electromagnetic spectrum Power of an electromagnetic wave
Power of an electromagnetic wave Electromagnetic spectrum nasa
Electromagnetic spectrum nasa Kesler science
Kesler science Electromagnetic spectrum foldable
Electromagnetic spectrum foldable Lesson 1 the electromagnetic spectrum
Lesson 1 the electromagnetic spectrum