Setting up a clinical research study in STH















- Slides: 18

Setting up a clinical research study in STH (how to get through the research governance process, and the support available) Respiratory Medicine Research Forum 4 th October 2013 jim. lithgow@sth. nhs. uk Respiratory Medicine Research Coordinator

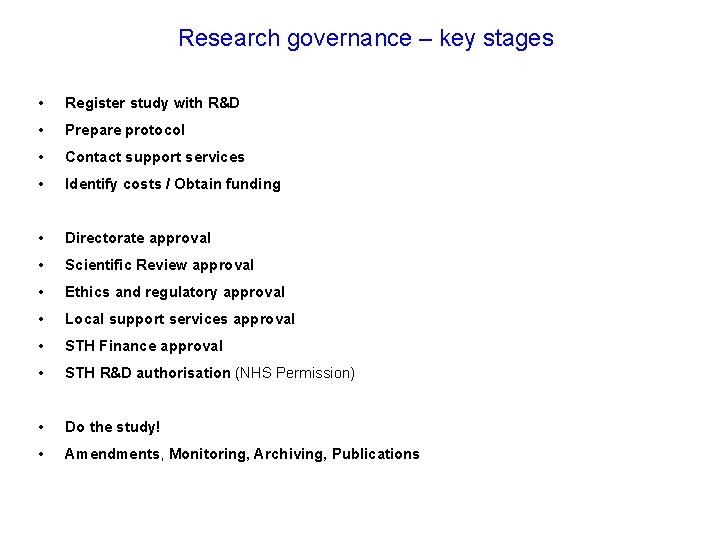
Research governance – key stages • Register study with R&D • Prepare protocol • Contact support services • Identify costs / Obtain funding • Directorate approval • Scientific Review approval • Ethics and regulatory approval • Local support services approval • STH Finance approval • STH R&D authorisation (NHS Permission) • Do the study! • Amendments, Monitoring, Archiving, Publications

Is your project "research"? • Clinical Audit “measures practice against evidence-based clinical standards". • Practice/Service Evaluation: “evidence-based evaluation of a service to inform local decision-making”. • Practice/Service Development: “introduction of a change in service delivery using evidence from research or other sources”. Ø Register with STH Clinical Effectiveness Unit: CAEU@sth. nhs. uk • Research “generates new knowledge where there is no or limited research evidence available and which has potential to be generalisable or transferable". Ø Register with STH R&D (see next slide) Tools to help decide: HRA Decision Tool: http: //www. nres. nhs. uk/applications/is-your-project-research/ Simple Rules Toolkit: http: //nww. sth. nhs. uk/STHcont. Docs/STH_CAE/Simple. Rules. Toolkit. pdf If in doubt, contact Research Coordinator and/or Clinical Effectiveness Unit

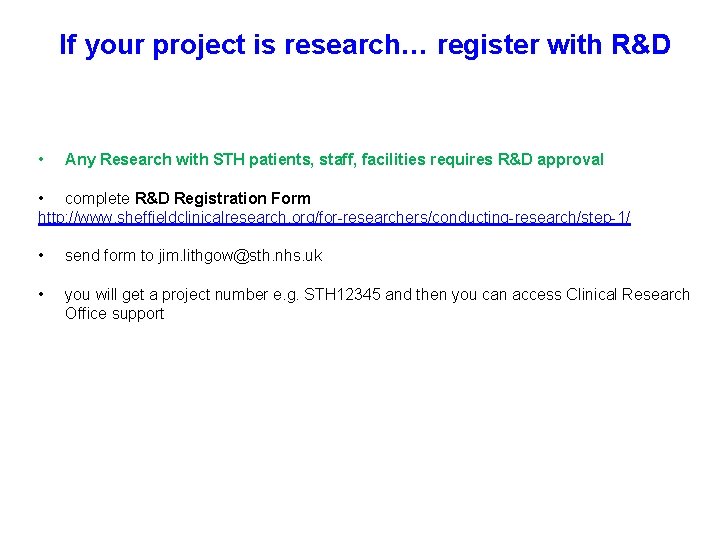
If your project is research… register with R&D • Any Research with STH patients, staff, facilities requires R&D approval • complete R&D Registration Form http: //www. sheffieldclinicalresearch. org/for-researchers/conducting-research/step-1/ • send form to jim. lithgow@sth. nhs. uk • you will get a project number e. g. STH 12345 and then you can access Clinical Research Office support

Clinical Research support in Sheffield (1) STH Respiratory Medicine • • Directorate Research Coordinator: jim. lithgow@sth. nhs. uk Research Development Officer: catherine. billings@sth. nhs. uk Clinical Research Office • CRO is a collaboration between STH and University of Sheffield http: //www. sheffieldclinicalresearch. org/ • STH R&D provides research governance advice and support, and ensures studies adhere to Research Governance Framework. STH R&D issue “NHS permission” for research. • STH R&D Coordinator for Respiratory studies: nana. theodorou@sth. nhs. uk Clinical Research Facility • A specialist environment for conduct of clinical research at RHH and NGH. Specialist research nurses and support staff provide a service tailored to each study. CRF is funded in partnership by National Institute for Health Research, STH and University of Sheffield: www. shef. ac. uk/faculty/medicine-dentistry-health/crf/home

Clinical Research support in Sheffield (2) http: //www. sheffieldclinicalresearch. org/for-researchers/training/ CRO summary of training available in Sheffield www. shef. ac. uk/faculty/medicine-dentistry-health/crf/educationandtraining CRF programme of education and training www. rds-yh. nihr. ac. uk/ NIHR Research Design Service supports researchers to design proposals for submission to NIHR and other national funders www. sheffield. ac. uk/scharr/sections/dts/ctru University of Sheffield Clinical Trials Research Unit supports design and conduct of clinical trials, and observational and cohort studies www. shef. ac. uk/scharr/shortcourseunit University of Sheffield, SCHARR: Short Course Unit www. shef. ac. uk/faculty/medicine-dentistry-health/thinkahead University “Think Ahead” programme of research training and mentoring, some courses open to STH staff. http: //www. crn. nihr. ac. uk/yorkshire-and-humber/ Yorkshire & Humber Clinical Research Network supports studies that are on the NIHR Portfolio (see later slide); also various training events. GCP (Good Clinical Practice): A key requirement for anyone involved in the conduct of clinical research, GCP is the standard and guidelines to which all research is conducted. Free courses or online training can be arranged by contacting jim. lithgow@sth. nhs. uk Statistics advice: STH R&D arrange stats clinics for studies registered with R&D. Contact aneeza. lone@sth. nhs. uk Research Funding: An email newsletter providing information on national research funding opportunities is available from the University of Sheffield. Contact k. c. williams@sheffield. ac. uk

Contractual requirements, GCP & CV Contracts, Letters of Access, Research Passports If you have an STH substantive or honorary contract, or are a healthcare placement student undertaking research, you have the required contract status for doing research in STH. If you have an NHS contract from another Trust, you may need a Letter of Access. If you are not in any of the above categories you need a Research Passport. If you require a Letter of Access or Research Passport, contact gaurika. kapoor@sth. nhs. uk. Start applying ASAP as Research Passports could take up to 8 weeks. GCP: “Good Clinical Practice” GCP is the standard and guidelines to which all clinical research is conducted. GCP training is a regulatory necessity for investigators in Drug and Device studies. The CRO recommend that all researchers complete a GCP course. Free courses or online training can be arranged, contact jim. lithgow@sth. nhs. uk CV: A curriculum vitae (max 2 pages preferred) for each member of study team should be sent to R&D.

Protocol: practical requirements to think about • Use STH R&D protocol template: http: //www. sheffieldclinicalresearch. org/for-researchers/usefuldocuments/forms-and-guidance/ • Get statistical advice. Has sample size enough “power”? • What is recruitment strategy and is target feasible? • Identification and approach of patients by should be by someone from patient’s clinical care team. • Patient Information Sheet - explain study and risks however small (template available). • An appropriately trained individual should obtain written consent from patients using REC-approved Consent Form (template available). • Patient and Public Involvement in design and conduct of research: consider PPI Panel. • Data Protection: all studies need STH Information Governance approval. Spell out arrangements for data storage, security, anonymisation, who will have access, transfer to other sites. • Describe collection, storage or use of Tissue for research. EITHER NHS REC approval, OR an HTA licence to store tissue for research purposes e. g. tissue bank. • Study Management: will there be Trial Steering Group / Data Monitoring Committee? Even if just two people - explain how often you will meet and responsibilities of study team. • Safety Reporting: explain “adverse events” reporting arrangement. There are guidelines about which events need reporting to R&D / REC / MHRA. • Insurance / Indemnity: NHS studies are covered by NHS Indemnity; in some cases other Insurance may be needed e. g. University of Sheffield Clinical Trials Insurance.

Identify the costs… how is it funded? • Start thinking about finance early in the process… • Are activities part of a patient's "routine clinical care“ OR additional “research” activities? Identify funding source(s) for all activities. • How much Chief / Principal Investigator / Supervisor involvement and how funded (e. g. 1 hr/wk, 2 days/wk)? • STH Support services: Pharmacy, Labs, CRF etc. have “research request forms”. Research Coordinators help you complete the forms. Each Department reviews capacity, capability, provides price. • Host Directorate costs? : – nurses / other staff – identification, screening, recruiting, consenting of patients – study visits, questionnaires, interviews, transcription – printing, postage, admin – patient / participant expenses • External providers: e. g. purchasing equipment, test kits, independent advice, IT etc. ? After identifying all costs, you apply for the required funding – Contact Research Coordinator and R&D to assist with grant applications ASAP. Alternatively, funding may already be available e. g. Directorate personal research funds.

Scientific Review and Sponsorship requirements Scientific review evidence allows organisations to be satisfied with scientific quality, strategic relevance and VFM, and is required as a condition of • • Do. H Research Governance Framework NHS Research Ethics approval STH Trust Sponsorship (see below) STH R&D authorisation • For grant-funded research, scientific review evidence can usually be obtained from the Funder. Alternatively, STH R&D can arrange independent review. • STH Clinical Research Facility studies are subject to additional CRF scientific and feasibility review. “Sponsorship” • Sponsor is organisation with responsibility for securing arrangements to initiate, manage and finance a study, e. g. an NHS Trust, a University, or a Commercial company. N. B. “Sponsor” does not just mean “Funder” • A Sponsor representative needs to sign various application forms. E. g. for STH-sponsored studies, STH R&D Manager signs Ethics submissions, and Director of R&D signs contracts and agreements.

Ethics: what type of ethics approval do I need? When R&D confirm the Scientific Review is appropriate and funding is confirmed, you are ready to submit for Ethical review. Research Ethics is about protecting the rights, safety, dignity and well-being of research participants It is best to assume you need NHS Research Ethics Committee review. See http: //www. nres. nhs. uk/ However NHS REC review is not needed where research involves: § previously collected, non-identifiable information from clinical care, anonymised to researchers § previously collected, non-identifiable tissue samples, with donor consent and HTA licence § previously collected acellular material (e. g. serum) from clinical care that is anonymised to researchers. Exception: NHS REC approval needed for DNA analysis without consent § staff recruited as research participants by virtue of their professional role. If your project does not need NHS REC review, it may need to be reviewed by a University Research Ethics Committee, and you still need R&D approval. NHS REC review applications are made using the online IRAS system, described in the next slide. Research Coordinator can assist you with this process.

National regulatory requirements and how to apply using IRAS: Integrated Research Application System: get an account at www. myresearchproject. org. uk • You complete online forms and transfer them to Research Coordinator. • Study information is entered into the system. It “autopopulates” different forms for: • • NHS REC MHRA (for some drug/device studies) NHS R&D Form: used by R&D for all studies NHS SSI Form: Site-Specific Information for each Participating Site (even if there is only one site!) • IRAS also includes information on: • Ionising Radiation and Nuclear Medicine e. g. for research involving X-ray, CT scans, etc • Research involving Human Tissue. • Study Amendments • IRAS forms need to be electronically signed by CI, Academic Supervisor, and STH R&D Manager. • Before you get signatures and submit forms, you must finalise all study documents, CVs, scientific review etc. • When the above is complete: telephone Central or Local REC to book a review date. • REC applications are submitted online using IRAS system • Advisable to attend REC meeting in person. • NHS REC is committed to returning an opinion within 60 days of receipt of a Valid application. • Low-risk studies eligible for Proportionate Review - reviewed within 14 days of receipt of valid application

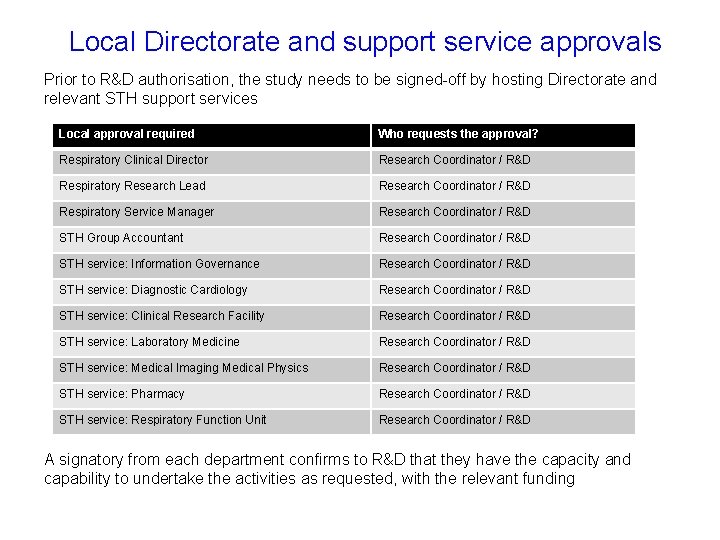
Local Directorate and support service approvals Prior to R&D authorisation, the study needs to be signed-off by hosting Directorate and relevant STH support services Local approval required Who requests the approval? Respiratory Clinical Director Research Coordinator / R&D Respiratory Research Lead Research Coordinator / R&D Respiratory Service Manager Research Coordinator / R&D STH Group Accountant Research Coordinator / R&D STH service: Information Governance Research Coordinator / R&D STH service: Diagnostic Cardiology Research Coordinator / R&D STH service: Clinical Research Facility Research Coordinator / R&D STH service: Laboratory Medicine Research Coordinator / R&D STH service: Medical Imaging Medical Physics Research Coordinator / R&D STH service: Pharmacy Research Coordinator / R&D STH service: Respiratory Function Unit Research Coordinator / R&D A signatory from each department confirms to R&D that they have the capacity and capability to undertake the activities as requested, with the relevant funding

Contracts, Finance sign-off, and NHS Permission from STH R&D STH Research Finance reviews all costs and how they will be funded, and must give “finance approval”. STH R&D will review requirements for any contracts or agreements, e. g. • • • research funding contracts agreements with providers of equipment or services Site Agreements for multi-centre studies Commercial Contracts arrangements with University (Side Letters) When you have ethical and regulatory approval, local Directorate and support service approval, contracts and STH Finance approval in place, R&D will issue a letter of NHS Permission for research!

Doing the study: what happens next? • Site Files maintained in a standard format, see R&D guidance. • Keep in touch with R&D to notify about recruitment, any issues etc. • Declaration of Helsinki states: "Every clinical trial must be registered in a publicly accessible database before recruitment of the first subject. " This does not include educational studies below doctoral level. Contact Research Coordinator for advice. • Amendments: inform R&D and NHS REC of any changes in the way you intend to perform the study. – Substantial Amendments need to be approved by NHS REC and R&D – Non-substantial Amendments require only that NHS REC and R&D are notified. – Amendment Forms are created within IRAS, and signed by CI and R&D. • Annual Progress Reports required by REC and MHRA, cc. to R&D (simple but useful) • Monitoring / Audit: R&D may require to periodically audit your Site File to ensure the study is being performed safely and appropriately - usually only STH-sponsored drug/device studies. • Archiving: Investigators should arrange appropriate archiving of study files and information: usually for 5 yrs after end of study. STH R&D arranges external archiving with Cintas Storage (£ 150 per box for 10 years). • Hopefully you will be able to publish the results of your study. Please let R&D know about publications and any outcomes of the research, such as changes to clinical practice etc.

NIHR Portfolio: how does it help and how do I get it? National Institute for Health Research provides funds for infrastructure, facilities, centres, grants and individual fellowships to conduct health research focused on the needs of patients. The Clinical Research Network (CRN) is the clinical research delivery arm of the NHS. They operate nationally across England through a national coordinating centre and 15 local branches delivering research in the NHS. What is the NIHR Portfolio? A database of high-quality studies that benefit from infrastructure and financial support. • supports Research Nurses, support staff, data collection and use of facilities, tests, or services where research activity adds value to patient care. • studies must satisfy quality criteria, checked by R&D and local Network. The Coordinated System for gaining NHS Permission (CSP) stores documents and approvals online so multi-site studies can be authorised ASAP. • Directorates receive money per patient recruited, and Portfolio activity informs allocation of “strategic” research funding to NHS Trusts. R&D give priority to Portfolio studies. • Portfolio and Commercial studies are “Performance Managed” by NIHR, so it is essential that studies are initiated quickly and patients are recruited to Time and Target. How do you get Portfolio support? http: //www. crn. nihr. ac. uk/can-help/funders-academics/nihrcrn-portfolio/how-to-apply-for-clinical-research-network-support/ Studies must already have full funding. • Automatically eligible studies are those funded by NIHR, Government and NIHR non-commercial Partners where funding has been awarded via open competition, with high quality peer review and with clear value to NHS. • Potentially eligible are: – Investigator-initiated, commercial-collaborative studies – Certain other high quality studies Commercially-sponsored Portfolio studies • do not receive financial support from Portfolio; however, they receive research governance support and prioritisation thus encouraging collaboration between NHS and industry. Contact your Research Coordinator for advice

Advice and support is available! • Respiratory Directorate Research Coordinator: jim. lithgow@sth. nhs. uk • Respiratory Research Development Officer: catherine. billings@sth. nhs. uk Clinical Research Office • http: //www. sheffieldclinicalresearch. org/ • STH R&D Coordinator for Respiratory studies: nana. theodorou@sth. nhs. uk • Patients / Public general enquiries: getinvolved@sth. nhs. uk Clinical Research Facility • www. shef. ac. uk/faculty/medicine-dentistry-health/crf/home

Respiratory Medicine Why are we involved in clinical research? For further information about getting involved in clinical research email getinvolved@sth. nhs. uk