Science Making measurements Accuracy and Precision Accuracy vs



- Slides: 18

Science Making measurements Accuracy and Precision

Accuracy vs Precision 1 of 9 Home 4 When you make a measurement it is often necessary to determine how good that measurement is. 4 Imagine a machine filling cans of soda. The company wants to make sure that there are exactly 12 ounces of soda in every can. 4 The company needs to gather information about the accuracy and precision of the filling machine.

Accuracy vs Precision 2 of 9 4 Accuracy and precision tell us how good a measurement is. 4 The terms accuracy and precision often cause trouble because we tend to use them interchangeably. However, these words have very different meanings. 4 Accuracy tells how close the measurement is to the true value. Precision describes the repeatability of the measurement.

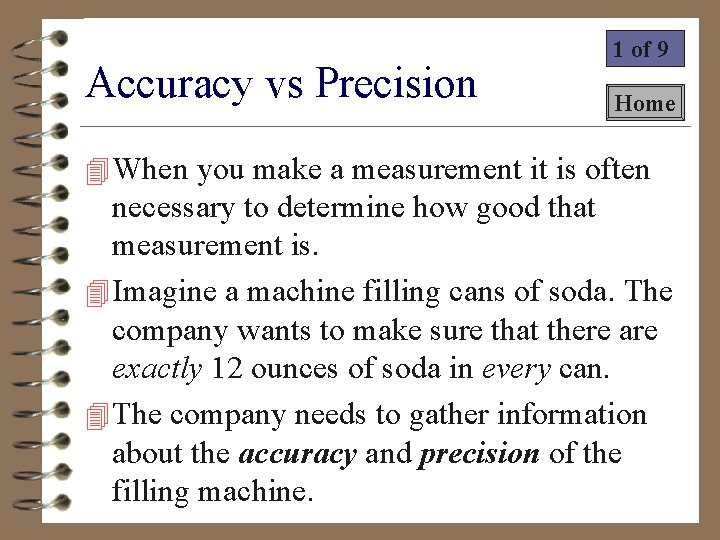
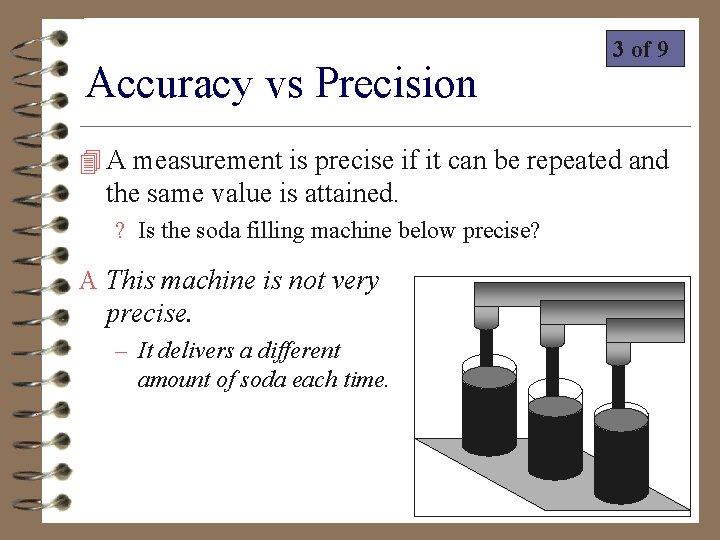
Accuracy vs Precision 3 of 9 4 A measurement is precise if it can be repeated and the same value is attained. ? Is the soda filling machine below precise? A This machine is not very precise. – It delivers a different amount of soda each time.

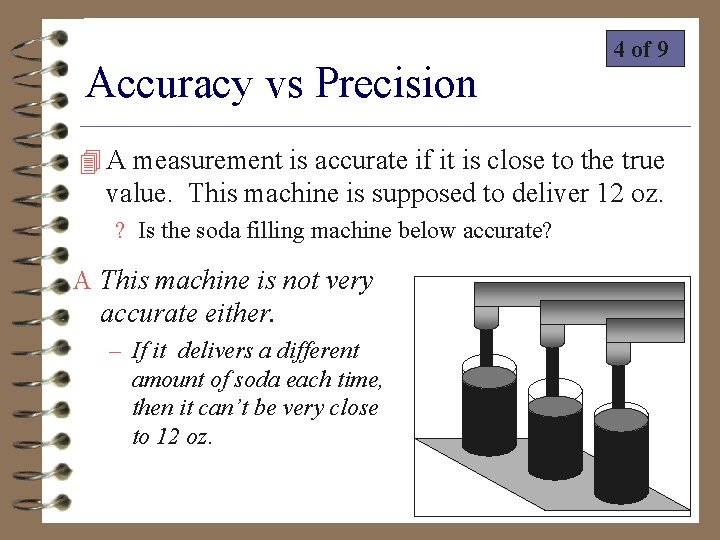
Accuracy vs Precision 4 of 9 4 A measurement is accurate if it is close to the true value. This machine is supposed to deliver 12 oz. ? Is the soda filling machine below accurate? A This machine is not very accurate either. – If it delivers a different amount of soda each time, then it can’t be very close to 12 oz.

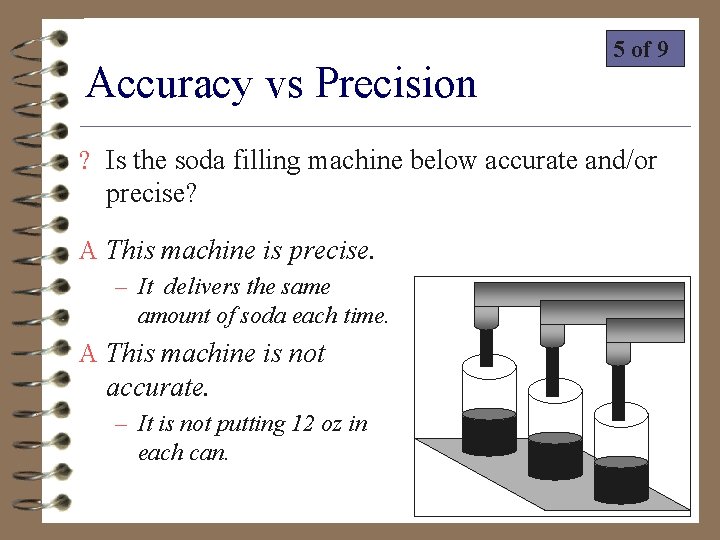
Accuracy vs Precision 5 of 9 ? Is the soda filling machine below accurate and/or precise? A This machine is precise. – It delivers the same amount of soda each time. A This machine is not accurate. – It is not putting 12 oz in each can.

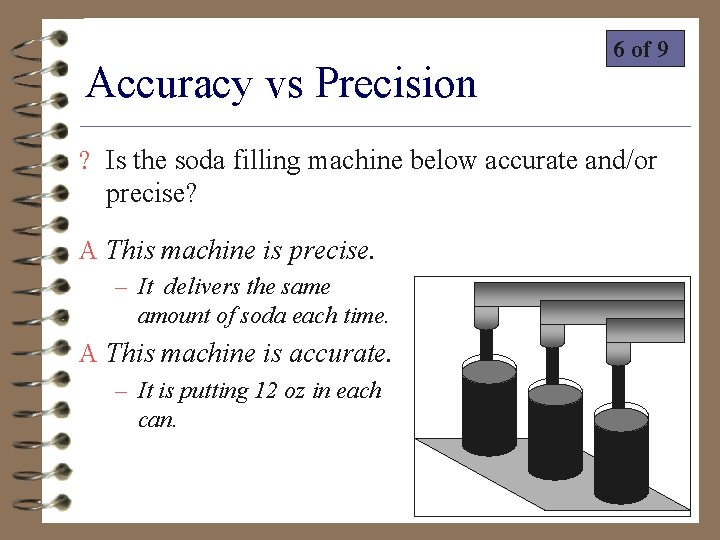
Accuracy vs Precision 6 of 9 ? Is the soda filling machine below accurate and/or precise? A This machine is precise. – It delivers the same amount of soda each time. A This machine is accurate. – It is putting 12 oz in each can.

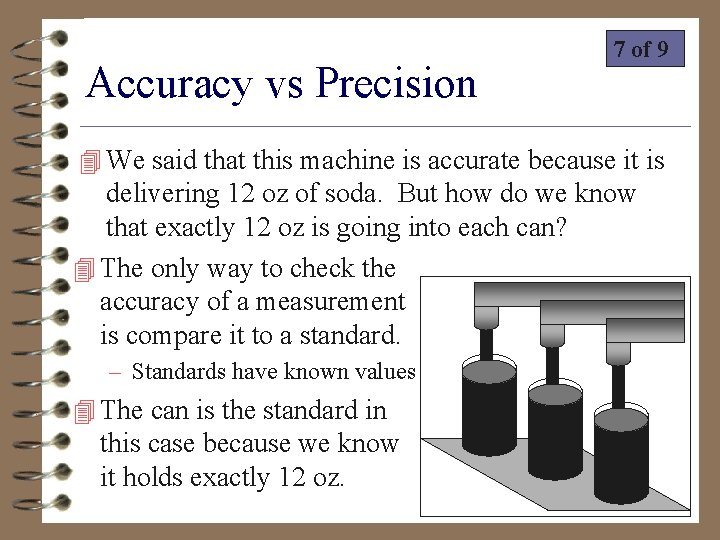
Accuracy vs Precision 7 of 9 4 We said that this machine is accurate because it is delivering 12 oz of soda. But how do we know that exactly 12 oz is going into each can? 4 The only way to check the accuracy of a measurement is compare it to a standard. – Standards have known values 4 The can is the standard in this case because we know it holds exactly 12 oz.

Accuracy vs Precision 8 of 9 ? Define accuracy and precision A Accuracy describes how close a measurement is to the true value. You need a standard to determine accuracy. A Precision describes how similar the values are each time you measure.

Accuracy vs Precision 9 of 9 Home ? How could you tell if the scale in the produce department of the supermarket is accurate and precise. A You could weigh a bunch of bananas three times. If you get the same value each time then the scale is precise. This doesn’t necessarily mean the scale is accurate. A The only way to check the accuracy of the scale is to use a standard. You need something of known weight. If you put a 1 pound package of butter on the scale and it reads 1 pound then the scale is accurate. The butter was used as a standard.

Science Measurements You have completed “Accuracy vs Precision”

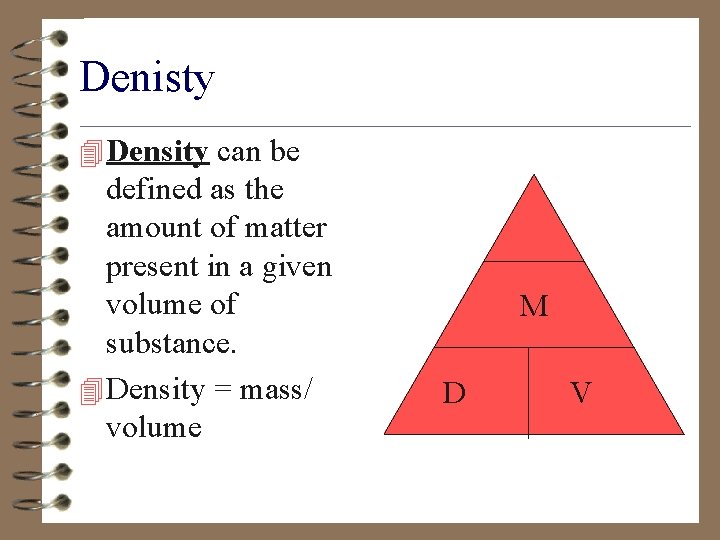
Denisty 4 Density can be defined as the amount of matter present in a given volume of substance. 4 Density = mass/ volume M D V

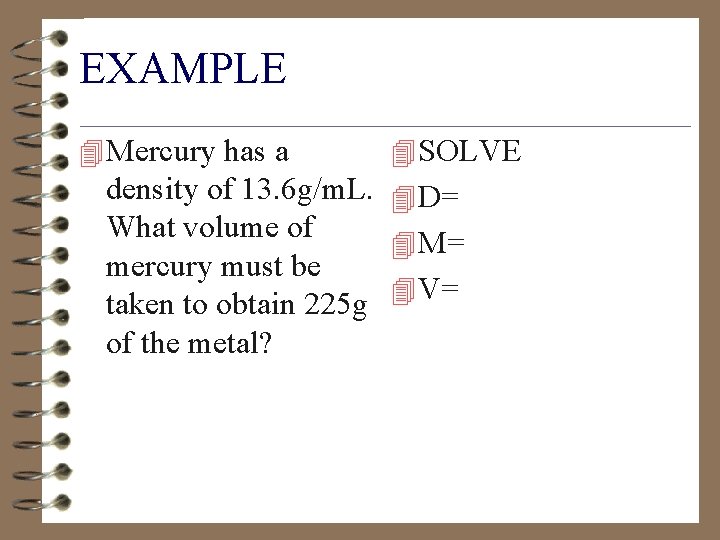
EXAMPLE 4 Mercury has a 4 SOLVE density of 13. 6 g/m. L. 4 D= What volume of 4 M= mercury must be 4 V= taken to obtain 225 g of the metal?

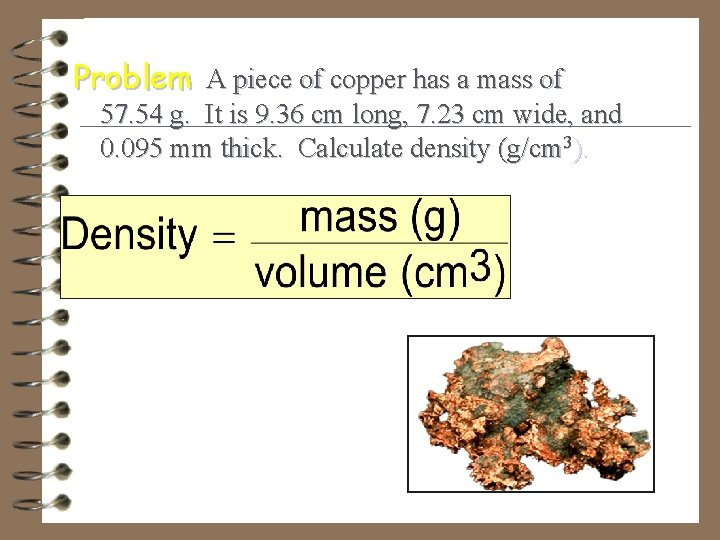
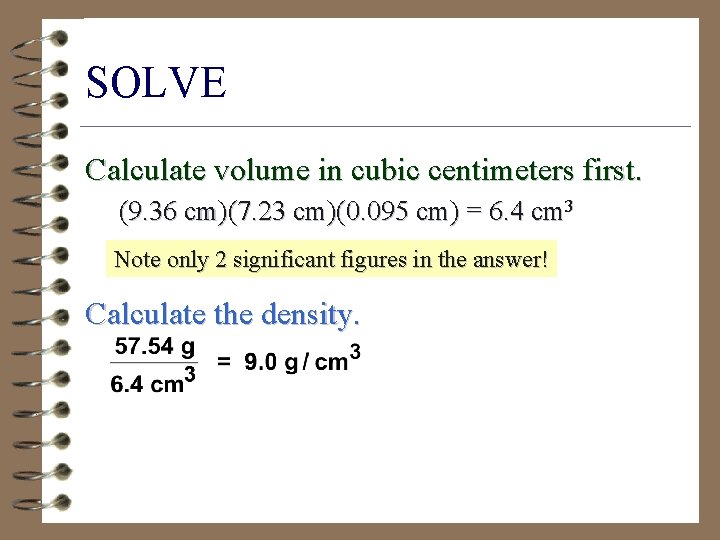
Problem A piece of copper has a mass of 57. 54 g. It is 9. 36 cm long, 7. 23 cm wide, and 0. 095 mm thick. Calculate density (g/cm 3).

SOLVE Calculate volume in cubic centimeters first. (9. 36 cm)(7. 23 cm)(0. 095 cm) = 6. 4 cm 3 Note only 2 significant figures in the answer! Calculate the density.

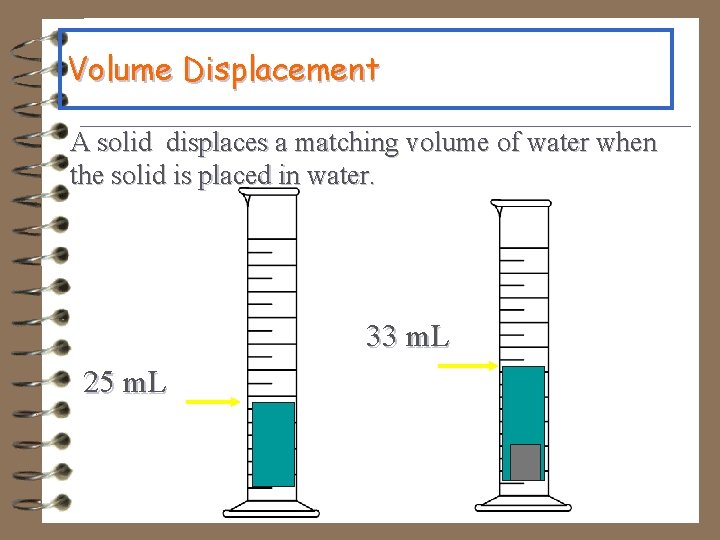
Volume Displacement A solid displaces a matching volume of water when the solid is placed in water. 33 m. L 25 m. L

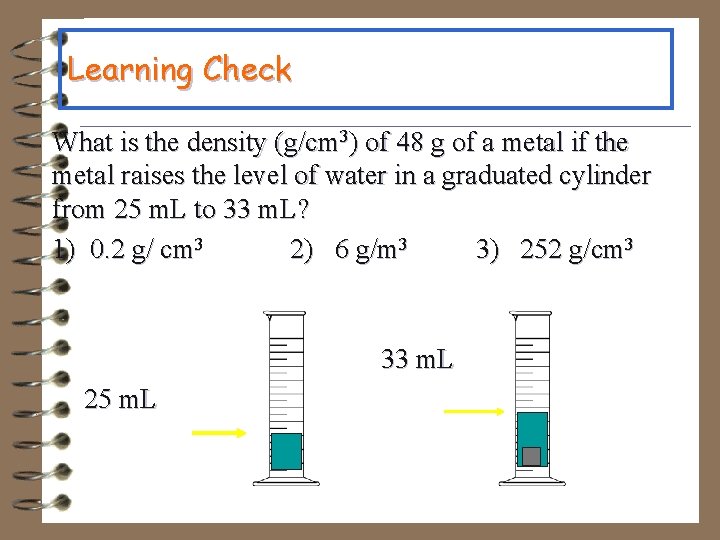
Learning Check What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? 1) 0. 2 g/ cm 3 2) 6 g/m 3 3) 252 g/cm 3 33 m. L 25 m. L
