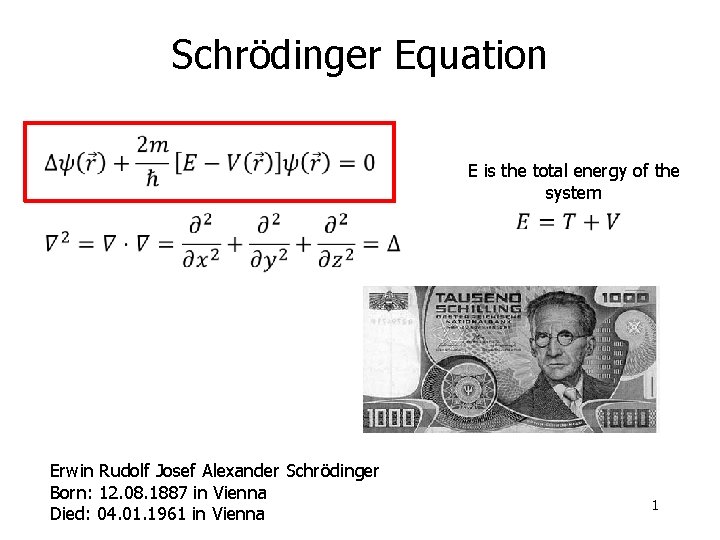
Schrdinger Equation E is the total energy of
![Formation of Energy Bands V II I II x a b k. I [cm-1] Formation of Energy Bands V II I II x a b k. I [cm-1]](https://slidetodoc.com/presentation_image_h/2a1f0a7b2018b8df80bbf83f3945c97f/image-8.jpg)
![Electron Energy Bands in Solids k. I [cm-1] Electronic structure of matters, i. e. Electron Energy Bands in Solids k. I [cm-1] Electronic structure of matters, i. e.](https://slidetodoc.com/presentation_image_h/2a1f0a7b2018b8df80bbf83f3945c97f/image-9.jpg)
- Slides: 13

Schrödinger Equation E is the total energy of the system Erwin Rudolf Josef Alexander Schrödinger Born: 12. 08. 1887 in Vienna Died: 04. 01. 1961 in Vienna 1

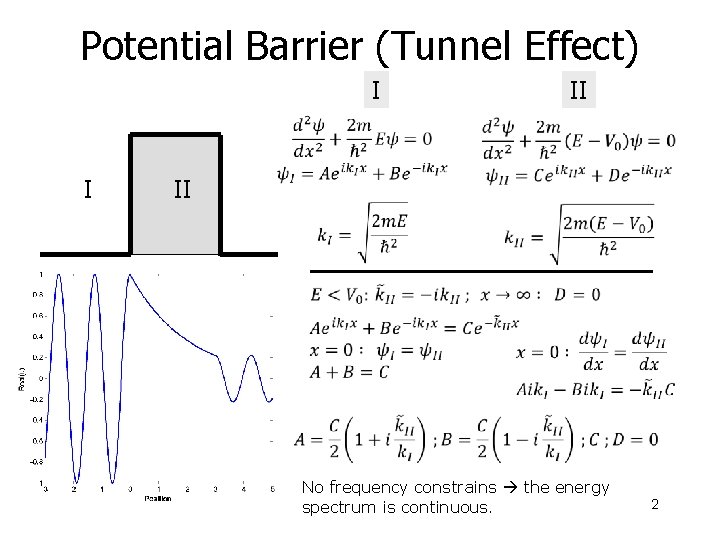
Potential Barrier (Tunnel Effect) I I II No frequency constrains the energy spectrum is continuous. 2

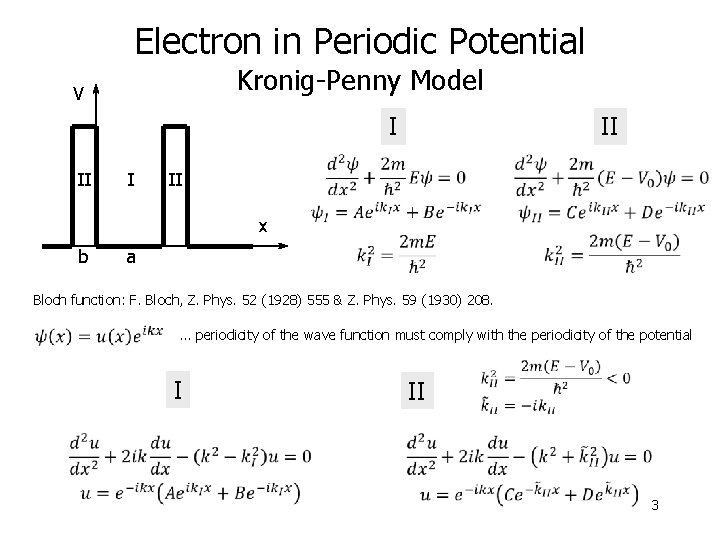
Electron in Periodic Potential Kronig-Penny Model V I II II x b a Bloch function: F. Bloch, Z. Phys. 52 (1928) 555 & Z. Phys. 59 (1930) 208. … periodicity of the wave function must comply with the periodicity of the potential I II 3

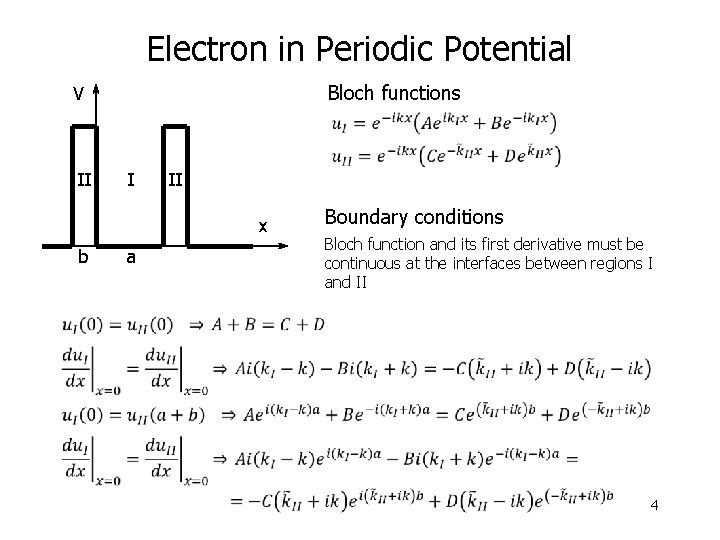
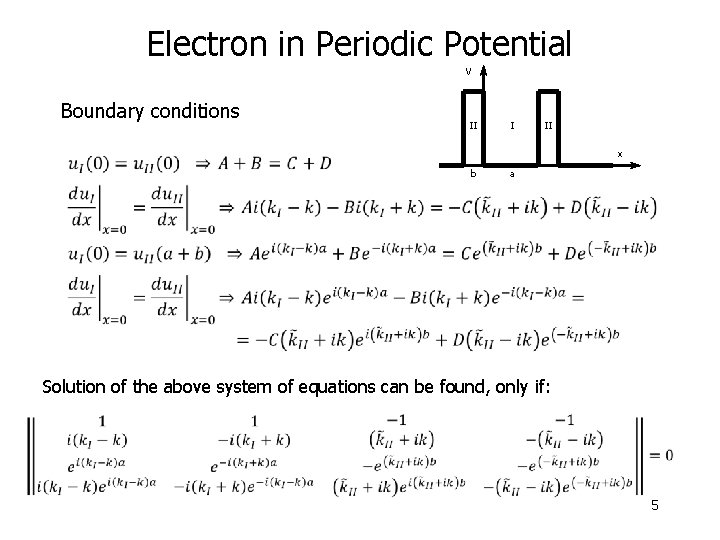
Electron in Periodic Potential Bloch functions V II I II x b a Boundary conditions Bloch function and its first derivative must be continuous at the interfaces between regions I and II 4

Electron in Periodic Potential V Boundary conditions II I II x b a Solution of the above system of equations can be found, only if: 5

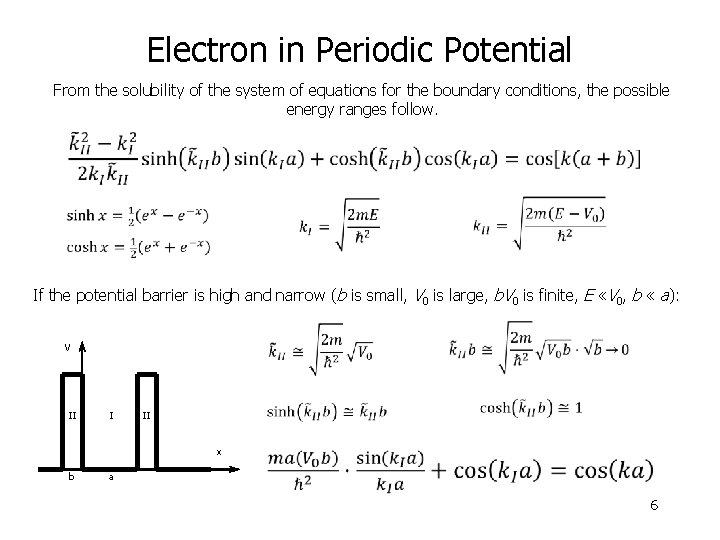
Electron in Periodic Potential From the solubility of the system of equations for the boundary conditions, the possible energy ranges follow. If the potential barrier is high and narrow (b is small, V 0 is large, b. V 0 is finite, E «V 0, b « a): II I a II x b V 6

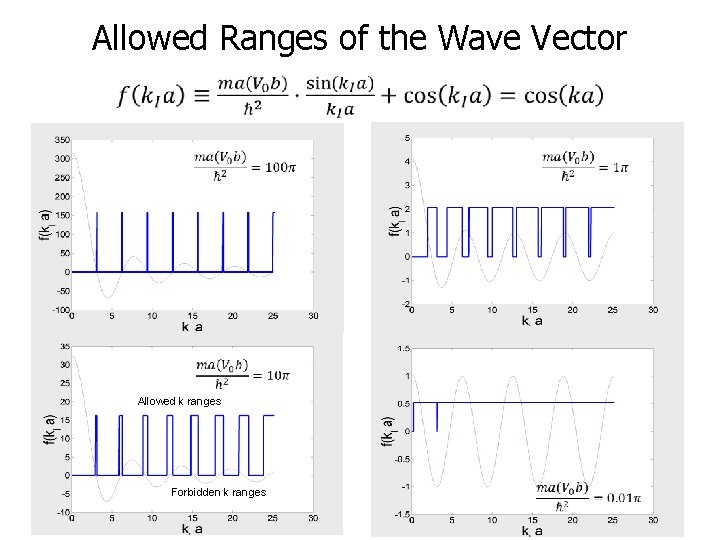
Allowed Ranges of the Wave Vector Allowed k ranges Forbidden k ranges 7
![Formation of Energy Bands V II I II x a b k I cm1 Formation of Energy Bands V II I II x a b k. I [cm-1]](https://slidetodoc.com/presentation_image_h/2a1f0a7b2018b8df80bbf83f3945c97f/image-8.jpg)
Formation of Energy Bands V II I II x a b k. I [cm-1] Parabolic dependence of energy on the wave vector However, only certain regions of the wave vectors are allowed. 8
![Electron Energy Bands in Solids k I cm1 Electronic structure of matters i e Electron Energy Bands in Solids k. I [cm-1] Electronic structure of matters, i. e.](https://slidetodoc.com/presentation_image_h/2a1f0a7b2018b8df80bbf83f3945c97f/image-9.jpg)
Electron Energy Bands in Solids k. I [cm-1] Electronic structure of matters, i. e. , the spectrum of the allowed wave vectors and energies, is given by the: Ø Strength of the potential barrier (V 0 b) Ø Periodicity of the lattice potential (a) Knowledge of the crystal structure and the kind of the chemical bonds are required. 9

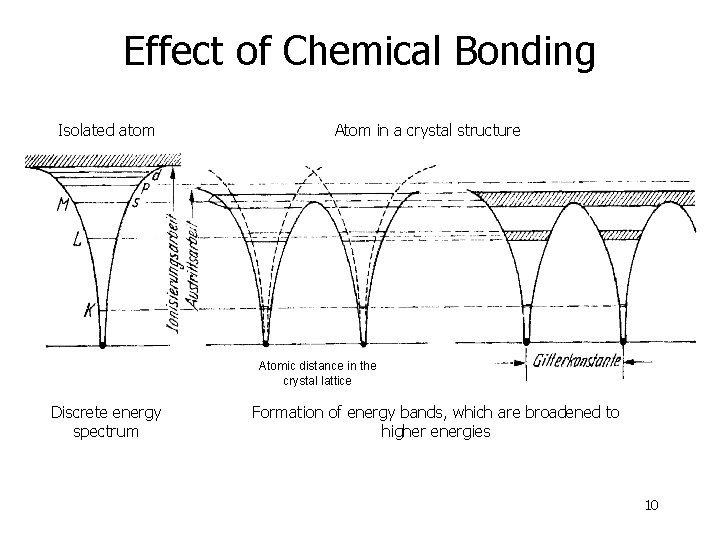
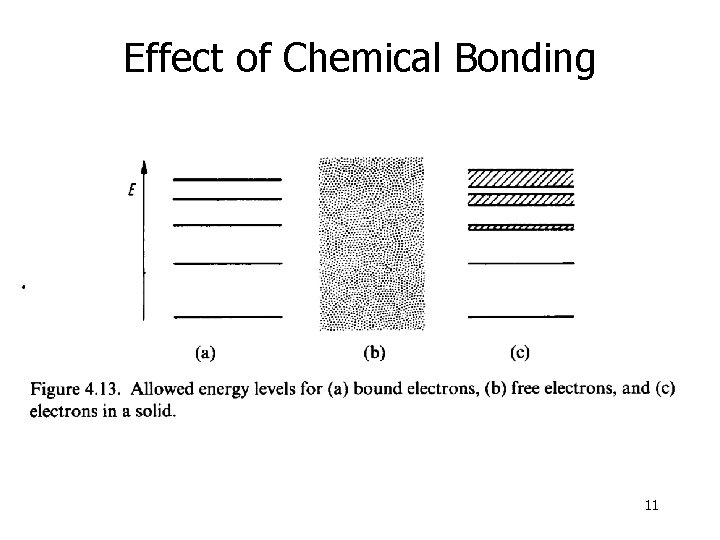
Effect of Chemical Bonding Isolated atom Atom in a crystal structure Atomic distance in the crystal lattice Discrete energy spectrum Formation of energy bands, which are broadened to higher energies 10

Effect of Chemical Bonding 11

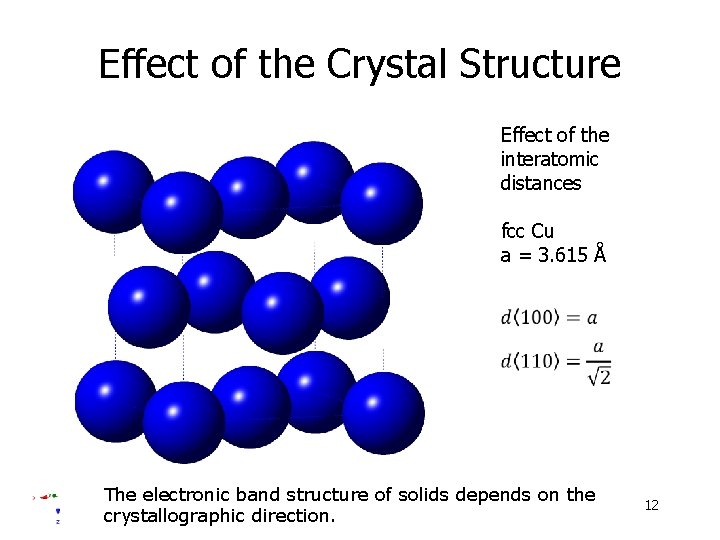
Effect of the Crystal Structure Effect of the interatomic distances fcc Cu a = 3. 615 Å The electronic band structure of solids depends on the crystallographic direction. 12

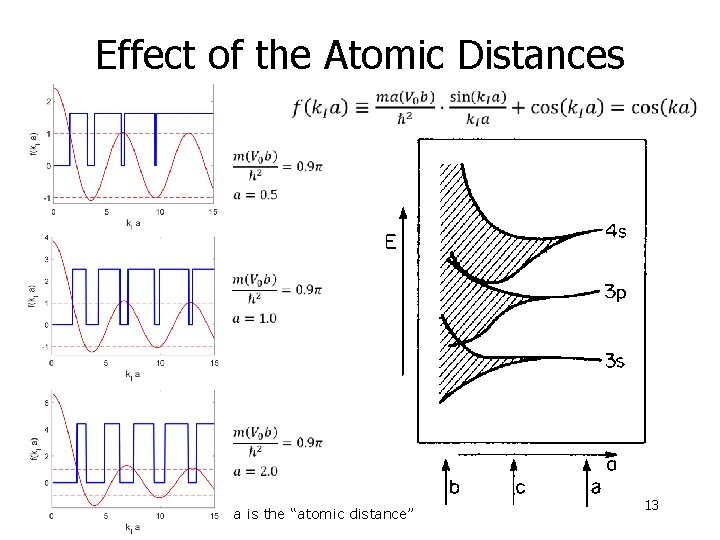
Effect of the Atomic Distances a is the “atomic distance” 13