Salinity Temperature Gasses Pressure OCEAN WATER CHEMISTRY WILL














- Slides: 16

Salinity Temperature Gasses Pressure OCEAN WATER CHEMISTRY

WILL THE EGG SINK OR FLOAT? Carefully observe the two containers on the lab table. Notice one is labeled fresh water and the other salt water Observe what happens to an uncooked egg when it is lowered into each container. Compare what happens to the two eggs. Answer the following— What does this tell you about the difference between salt water and fresh water?

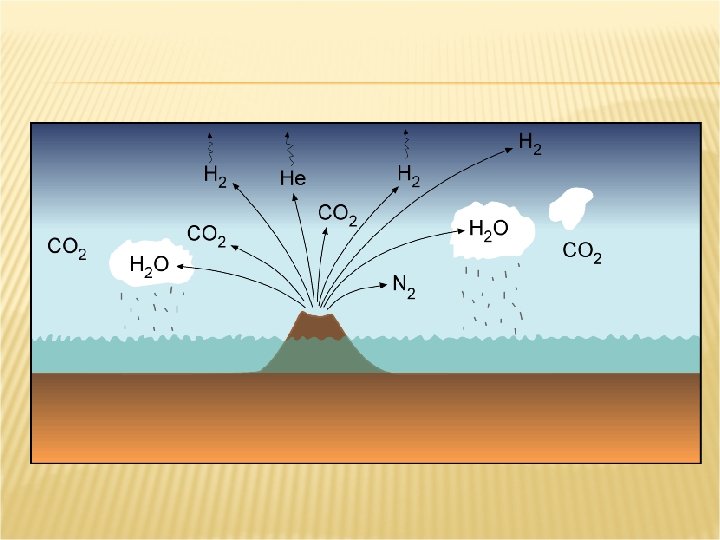
THE SALTY OCEAN In early stages of Earth’s history Ocean covered most of the surface Underwater volcanoes erupted releasing chemicals into water Land built up from eruptions Rain washed over land dissolving chemicals which ended up in the oceans Over time levels built up to present day amounts


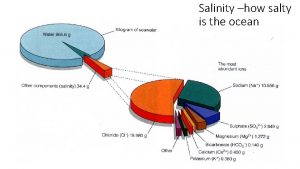
SALINITY Total amount of dissolved salts in water On average- 1 kilogram of ocean water contains 35 grams of salt Kilo = thousand Gram = measurement of substance Ocean water salinity is 35 g/1 kilogram 35 parts per thousand 3. 5% of 100%

COMPOSITION OF OCEAN WATER 95. 5% is water 3. 5% is dissolved salts…. . Of the remaining 1% 55% chlorine 30. 6% sodium 7. 7% sulfate 3. 7% magnesium 1. 2% calcium 1. 1% potassium 0. 7% other

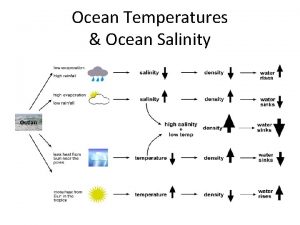
VARIATIONS IN SALINITY Most parts of ocean is between 34 to 37% Near surface – lower % due to addition of fresh water (rain, snow, melting ice) (Near mouth of rivers - lower due to addition of fresh water) Near poles – higher due to freezing of ice Fresh water freezes leaving behind salt concentrations Hot & dry climate regions – higher due to evaporation Fresh water evaporates leaving behind salt concentrations


EFFECTS OF SALINITY Ocean water freezes at -1. 9 o C Fresh water freezes at 0 o. C Salt acts like antifreeze by interfering with the formation of ice crystals structure Salt water has higher density Mass of same amount of salt water is greater than that of fresh water Means greater buoyancy (lifting up less dense objects floating )


TEMPERATURE OF OCEAN WATER Temperatures seasons Why? at surface vary with location and Broad surface area absorbs sun’s infrared energy. Greater amount of infrared energy levels = greater absorption = higher temperatures Warm water is less dense – thin layer at surface Colder, denser water – deeper you descend Molecules of water huddle as they get colder = more molecules per sample compared to warm water = cold water more dense


GASES IN OCEAN WATER Ocean organisms need & use the gases Carbon dioxide 60 times more than in air Algae needs for photosynthesis Coral animals need to build hard exoskeletons Oxygen Scarcer than carbon dioxide Most near surface Comes from the air and algae Affected by temperature Cold water (Polar) contains more oxygen than warm (Tropical)

CHANGES WITH DEPTH Decreasing temperature If temperature gets colder does the water at the bottom of the ocean freeze? Why or why not? 3 zones in the water column Surface Zone warmest -avg. 17. 5 o. C extends to 100 to 500 meters; avg. is 200 meters deep Transition bottom of surface zone to 1 kilometer temperatures drop rapidly as descend to about 4 o. C Deep Zone (Thermocline) Zone extends from 1 km to ocean floor avg. temp. 3. 5 o. C

CHANGES WITH DEPTH CONTINUED Increasing Pressure Water pressure is the force exerted by the weight of water Increases continually with depth Greater force due to greater amount of water above gravity pulls force downward Issue with high pressure in deep water Divers only safely descend to 40 meters Beyond 40 need submersible

CHANGES WITH DEPTH CONTINUED AGAIN Color and Light Sunlight penetrates the surface (during the day) First appears yellowish from sun’s visible light rays Then blue-green as red light is absorbed by the water No light reaches below 200 meters
 Salinity of ocean water ppt
Salinity of ocean water ppt How many elements are gasses at room temperature
How many elements are gasses at room temperature Water logging and salinity
Water logging and salinity Chapter 15 ocean water and ocean life
Chapter 15 ocean water and ocean life Water and water and water water
Water and water and water water Ocean water chemistry
Ocean water chemistry Are gasses highly compressible
Are gasses highly compressible Noble-gas notation
Noble-gas notation Gasses or gases
Gasses or gases Most weather reports for the general public use
Most weather reports for the general public use Gasses
Gasses Blue gasses
Blue gasses Properties of a gas
Properties of a gas Magma volatile gasses definition
Magma volatile gasses definition Global salinity map
Global salinity map Great salt lake salinity
Great salt lake salinity Messinian
Messinian