Related Quiz Questions Experimentally Determining Moles of Hydrogen









- Slides: 13

Related Quiz Questions Experimentally Determining Moles of Hydrogen

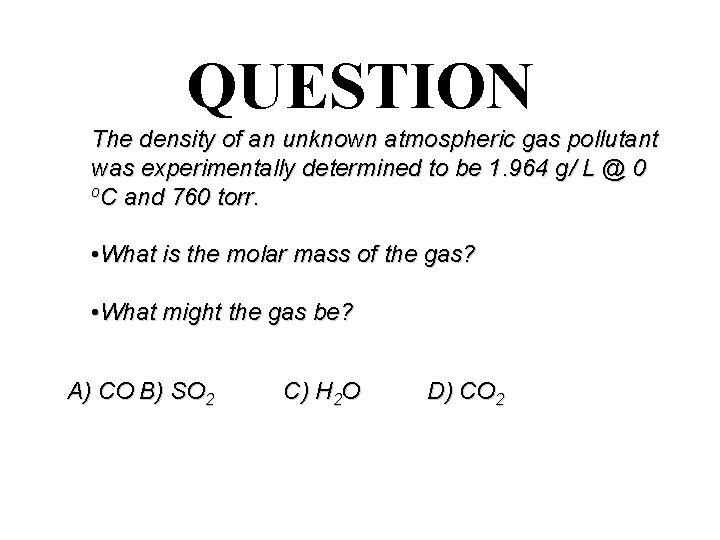
QUESTION The density of an unknown atmospheric gas pollutant was experimentally determined to be 1. 964 g/ L @ 0 o. C and 760 torr. • What is the molar mass of the gas? • What might the gas be? A) CO B) SO 2 C) H 2 O D) CO 2

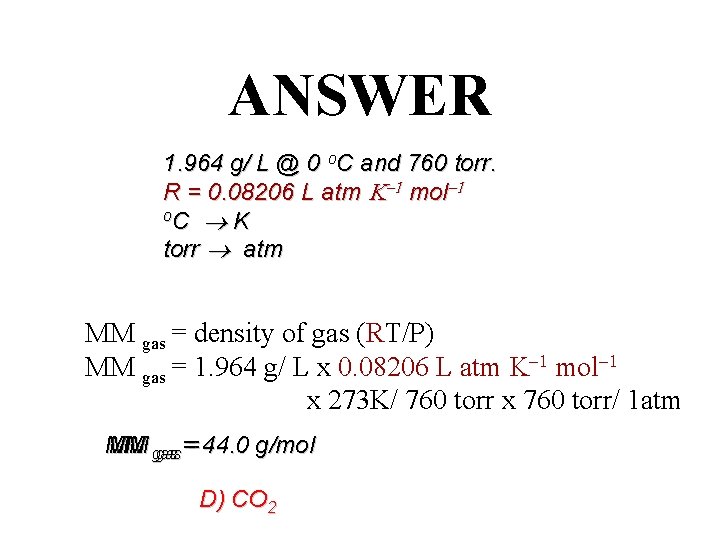
ANSWER 1. 964 g/ L @ 0 o. C and 760 torr. R = 0. 08206 L atm mol o. C K torr atm MM gas = density of gas (RT/P) MM gas = 1. 964 g/ L x 0. 08206 L atm mol x 273 K/ 760 torr x 760 torr/ 1 atm MM MM gas = 44. 0 g/mol gas= D) CO 2

QUESTION Freon-12, CF 2 Cl 2, a “safe” compressible gas, was widely used from 1935 -1994 as a refrigerant in refrigerators, freezers, and air conditioning systems. However, it had been shown to be a greenhouse gas and to catalytically destroy the ozone layer in a ratio of >14, 000: 1. It was phased out and banned. 200. ml of Freon-12 was collected by syringe. It weighed 0. 927 grams, had a temperature of 30. 0°C (303. 1 K), and a pressure of 730 mm of Hg (. What is the experimental molar mass of Freon-12? • • • 12. 1 g/mol 84 g/mol 92. 7 g/mol 115 g/mol 121. g/mol R = 0. 082 L atm K− 1 mol− 1

ANSWER E) 121 g/mol is the molar mass of Freon-12. MM gas = g of gas/V (RT/P)

QUESTION 0. 0820 grams of a volatile compound in the gas phase, which smells like fresh raspberries, was trapped in a syringe. It had a volume of 12. 2 m. L at 1. 00 atmosphere of pressure and 25. 0°C. What is the molar mass of this pleasant smelling compound ? A) B) C) D) 13. 8 g/mol 164 g/mol 40. 9 g/mol 224 g/mol

ANSWER B) 164 g/mol Using PV = n. RT : 0. 0122 L for V, 298 K for T, 0. 08206 for R and solving for n = 1. MOLAR MASS (grams in one mole) can be calculated. MM gas = density of gas (RT/P) MM gas = 0. 0820 g/ L x 0. 00122 L x 0. 0821 atm mol x 298 K/ 1 atm

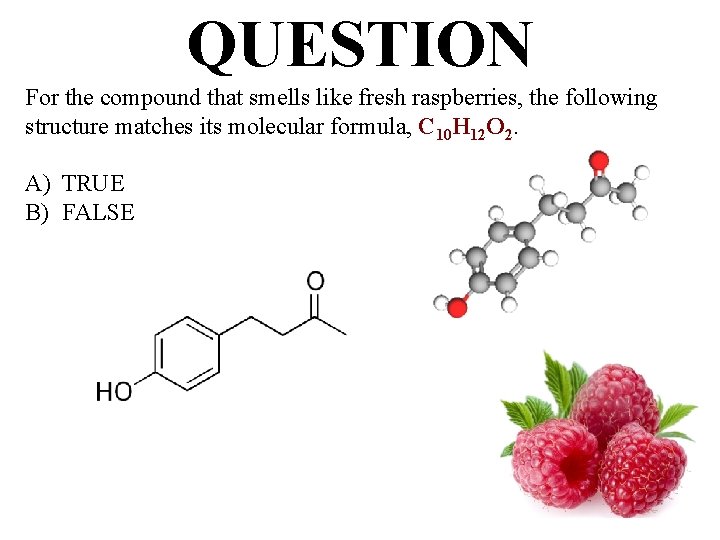
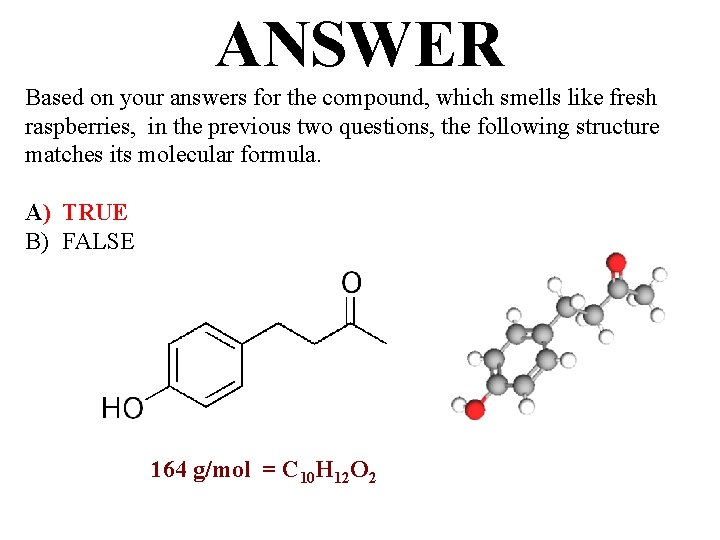
QUESTION For the compound that smells like fresh raspberries, the following structure matches its molecular formula, C 10 H 12 O 2. A) TRUE B) FALSE

ANSWER Based on your answers for the compound, which smells like fresh raspberries, in the previous two questions, the following structure matches its molecular formula. A) TRUE B) FALSE 164 g/mol = C 10 H 12 O 2

QUESTION Which sequence represents the gases in order of increasing density at STP? A) Fluorine < Carbon monoxide < Chlorine < Argon B) Carbon monoxide < Fluorine < Argon < Chlorine C) Argon < Carbon monoxide < Chlorine < Fluorine D) Fluorine < Chlorine < Carbon monoxide < Argon

ANSWER Which sequence represents the gases in order of increasing density at STP? A) Fluorine < Carbon monoxide < Chlorine < Argon B) Carbon monoxide < Fluorine < Argon < Chlorine C) Argon < Carbon monoxide < Chlorine < Fluorine D) Fluorine < Chlorine < Carbon monoxide < Argon

QUESTION Real gases exhibit their most “ideal” behavior at which relative conditions? A) B) C) D) Low temperatures and low pressures High temperatures and high pressures High temperatures and low pressures Low temperatures and high pressures

ANSWER C) High temperatures and low pressures At these conditions gas molecules are farthest apart and exert their least influence on each other (High kinetic energy and low potential energy) thereby permitting their behavior to more closely follow mathematical formulas, “laws’.
 Ideal gas law powerpoint
Ideal gas law powerpoint Project on congruence of triangles class 9
Project on congruence of triangles class 9 The integration of eye, hand, and foot movements
The integration of eye, hand, and foot movements Skill related fitness vs health related fitness
Skill related fitness vs health related fitness To atoms you say
To atoms you say Traditional methods for determining requirements
Traditional methods for determining requirements Earthquake p-wave and s-wave travel time graph
Earthquake p-wave and s-wave travel time graph End group analysis
End group analysis How to determine the rate determining step
How to determine the rate determining step What is the point estimate of μ?
What is the point estimate of μ? Assumptions of cvp analysis
Assumptions of cvp analysis Oxidation number rukes
Oxidation number rukes Activity 11-4 estimating time of death answer key
Activity 11-4 estimating time of death answer key Determining the optimal level of product availability
Determining the optimal level of product availability