Regulation for Aquaculture Medicine 8 July 2015 Dr








- Slides: 15

Regulation for Aquaculture Medicine 8 July 2015 Dr. Sasi Jaroenpioj Department of Livestock Medicine THAILAND

Regulations under Drug Act Pre-marketing Control • Licensing • Registration • Adversetisment Control Post-marketing Control • Licensee’ , Pharmacists’ and Drug store surveillance as mentioned in the Act • GMP Audit • Drug Surveillance • ADR Monitoring • Drug Reevaluation

List of licensed antimicrobials for use in aquatic animal in Thailand • • • 2/25/2021 Amoxycillin Enrofloxcin Sarafloxacin Oxolinic acid Oxytetracycline Sulfadimethoxine sodium / Ormethoprim Sulfadimethoxine sodium / Trimethoprim Sulfadiazine and Trimethoprim Sulfadimidine and Trimetroprim Sulfamonomethoxine Sodium Toltrazuril

List of banned antimicrobials in aquatic animal in Thailand • Chloramphenical • Nitrofuran and their Metabolites (Furazolidone, Furaltadone, Nitrofurazone and Nitrofurantoin) • Malachite green and Leuco-malachite green 2/25/2021

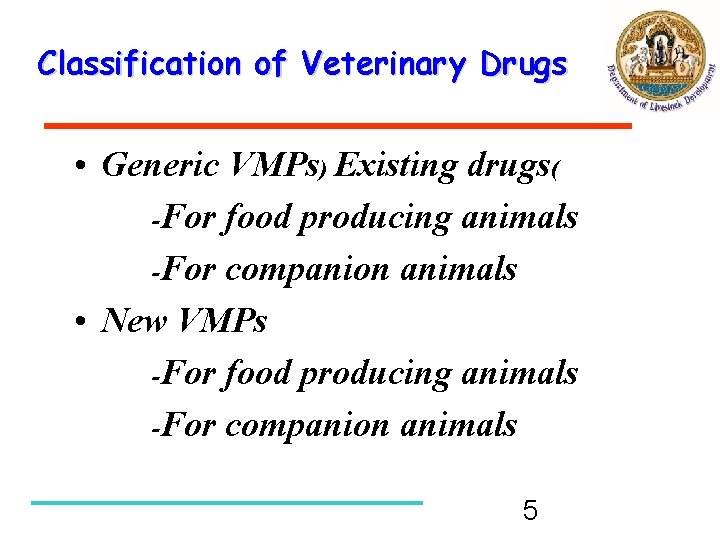
Classification of Veterinary Drugs • Generic VMPs) Existing drugs( -For food producing animals -For companion animals • New VMPs -For food producing animals -For companion animals 5

VMPs Registration Procedure 6

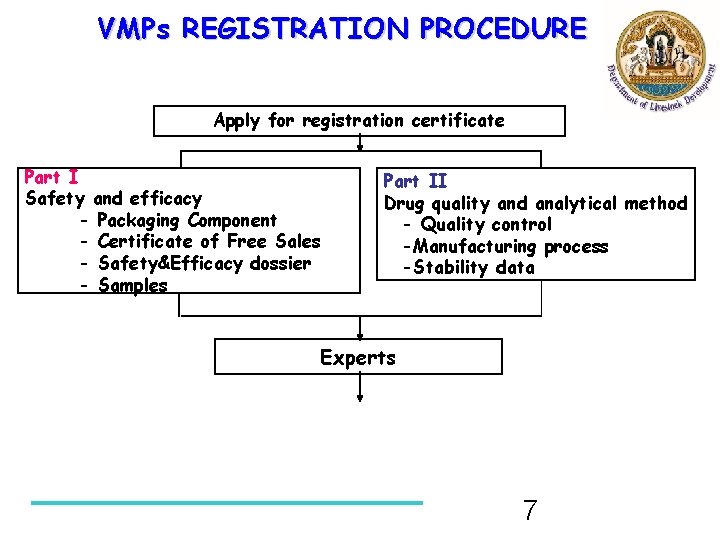
VMPs REGISTRATION PROCEDURE Apply for registration certificate Part I Safety and efficacy - Packaging Component - Certificate of Free Sales - Safety&Efficacy dossier - Samples Part II Drug quality and analytical method - Quality control -Manufacturing process -Stability data Experts 7

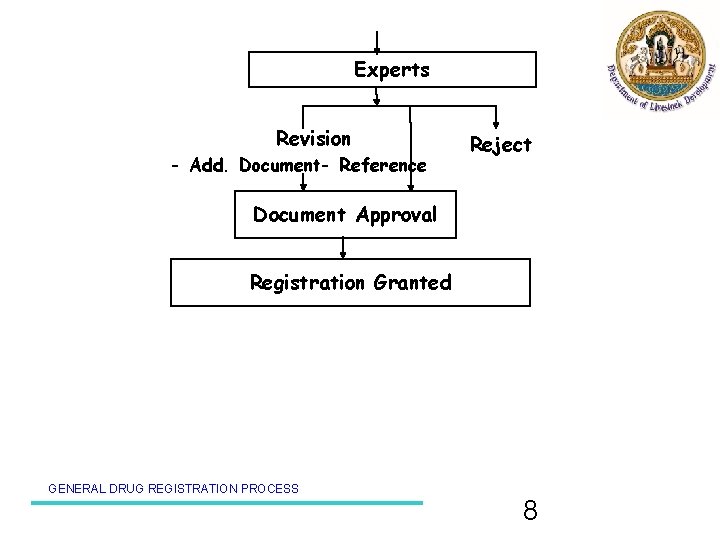
Experts Revision - Add. Document- Reference Reject Document Approval Registration Granted GENERAL DRUG REGISTRATION PROCESS 8

Document required for New VMPs for food producing spp. submission 9

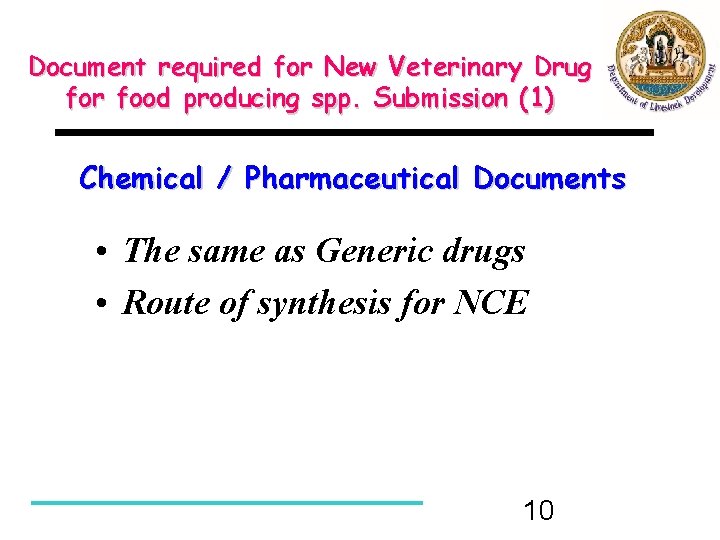
Document required for New Veterinary Drug for food producing spp. Submission (1) Chemical / Pharmaceutical Documents • The same as Generic drugs • Route of synthesis for NCE 10

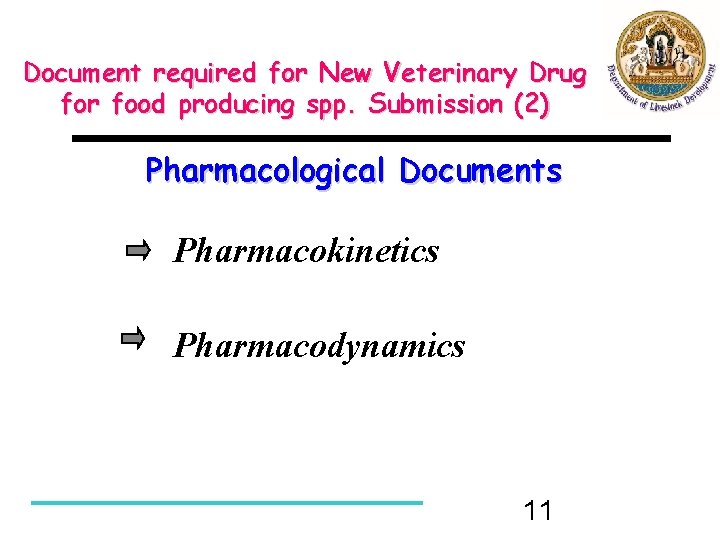
Document required for New Veterinary Drug for food producing spp. Submission (2) Pharmacological Documents Pharmacokinetics Pharmacodynamics 11

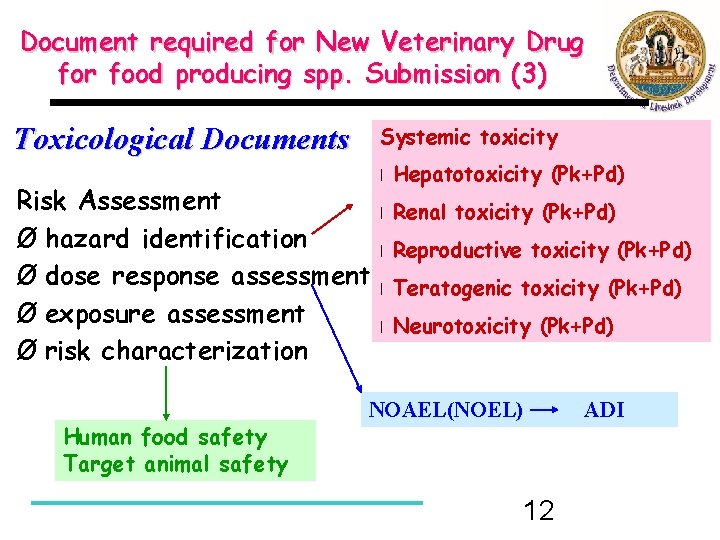
Document required for New Veterinary Drug for food producing spp. Submission (3) Toxicological Documents Systemic toxicity Risk Assessment Ø hazard identification Ø dose response assessment Ø exposure assessment Ø risk characterization Human food safety Target animal safety l Hepatotoxicity (Pk+Pd) l Renal toxicity (Pk+Pd) l Reproductive toxicity (Pk+Pd) l Teratogenic toxicity (Pk+Pd) l Neurotoxicity (Pk+Pd) NOAEL(NOEL) 12 ADI

Document required for New Veterinary Drug for food producing spp. Submission (4) Safety - Human Safety - Target Animal Safety - Environmental Safety - User Safety - The analytical method to determine the drug in edible tissue 13

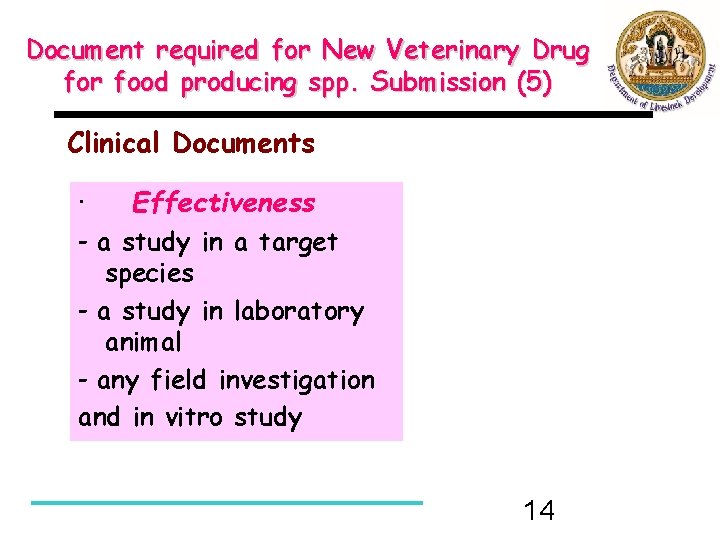
Document required for New Veterinary Drug for food producing spp. Submission (5) Clinical Documents · Effectiveness - a study in a target species - a study in laboratory animal - any field investigation and in vitro study 14

Thank you
 Bell aquaculture
Bell aquaculture Recirculating aquaculture system
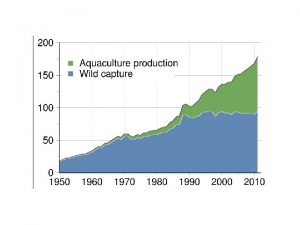
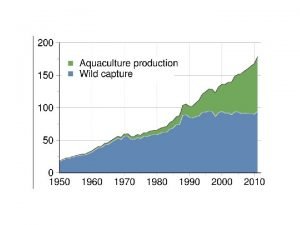
Recirculating aquaculture system Objectives of aquaculture
Objectives of aquaculture Process of aquaculture
Process of aquaculture Aquaculture doha
Aquaculture doha Aquaculture pest analysis
Aquaculture pest analysis Amanda alexandra
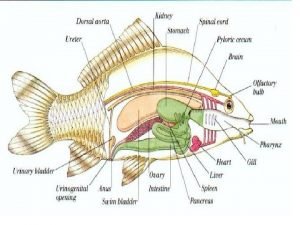
Amanda alexandra Characteristics of aquaculture
Characteristics of aquaculture Aquaculture funding opportunities
Aquaculture funding opportunities Aquaculture case study
Aquaculture case study New brunswick aquaculture
New brunswick aquaculture What are the objectives of aquaculture
What are the objectives of aquaculture Alabama marine resources division
Alabama marine resources division Flow-through systems
Flow-through systems Organic tilapia farming
Organic tilapia farming Maksud akuakultur
Maksud akuakultur