Reduced Viremia Following Immunotherapy of SIV with Peptide

- Slides: 17

Reduced Viremia Following Immunotherapy of SIV with Peptide Pulsed Blood Overlapping Peptide-pulsed Autologous Leukocytes Robert De Rose, University of Melbourne, Australia

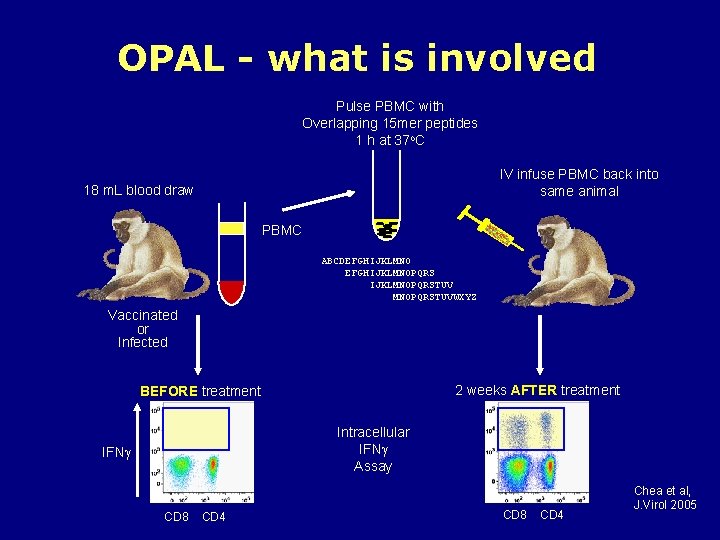
OPAL - what is involved Pulse PBMC with Overlapping 15 mer peptides 1 h at 37 o. C IV infuse PBMC back into same animal 18 m. L blood draw PBMC ABCDEFGHIJKLMNOPQRS IJKLMNOPQRSTUVWXYZ Vaccinated or Infected 2 weeks AFTER treatment BEFORE treatment Intracellular IFN Assay IFN CD 8 CD 4 Chea et al, J. Virol 2005

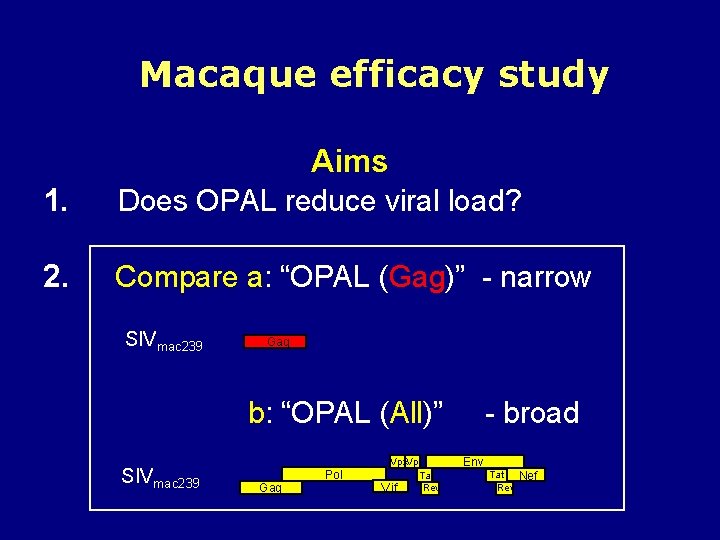
Macaque efficacy study Aims 1. Does OPAL reduce viral load? 2. Compare a: “OPAL (Gag)” - narrow SIVmac 239 Gag b: “OPAL (All)” SIVmac 239 Gag Pol Vpx. Vpr Tat tat Rev Vif - broad Env Tat Rev Nef

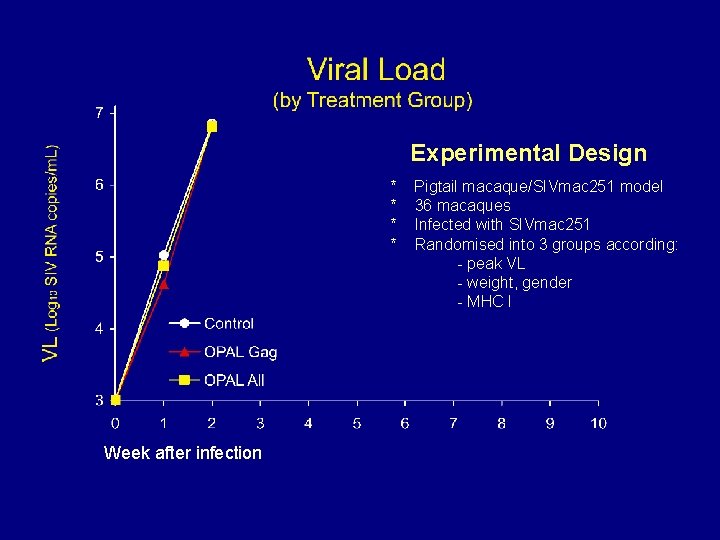
Experimental Design * * Week after infection Pigtail macaque/SIVmac 251 model 36 macaques

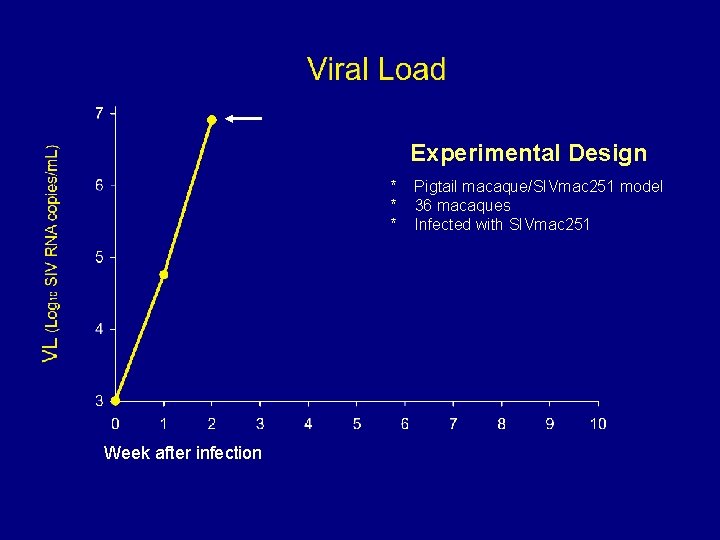
Experimental Design * * * Week after infection Pigtail macaque/SIVmac 251 model 36 macaques Infected with SIVmac 251

Experimental Design * * Week after infection Pigtail macaque/SIVmac 251 model 36 macaques Infected with SIVmac 251 Randomised into 3 groups according: - peak VL - weight, gender - MHC I

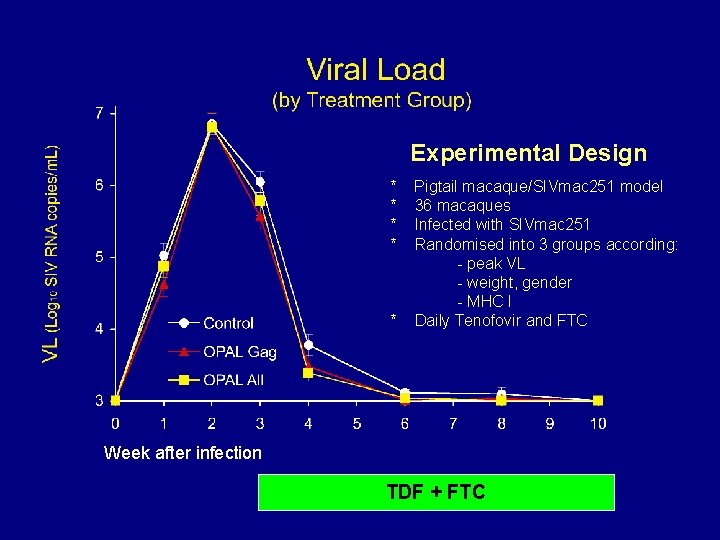
Experimental Design * * * Pigtail macaque/SIVmac 251 model 36 macaques Infected with SIVmac 251 Randomised into 3 groups according: - peak VL - weight, gender - MHC I Daily Tenofovir and FTC Week after infection TDF + FTC

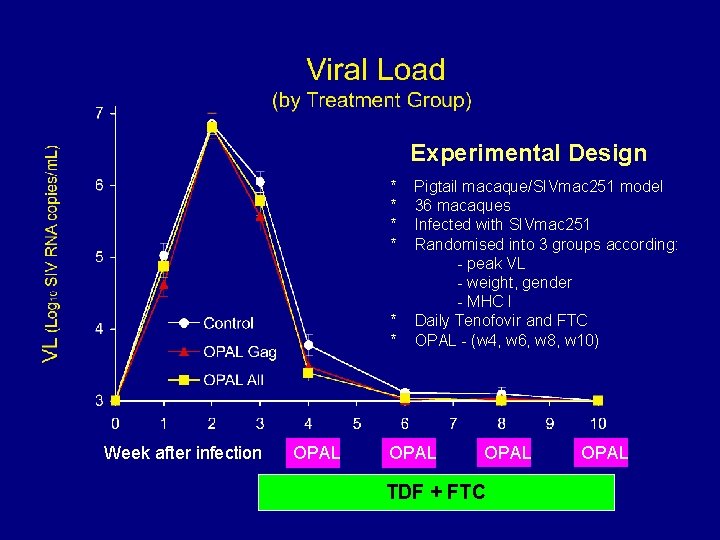
Experimental Design * * * Week after infection OPAL Pigtail macaque/SIVmac 251 model 36 macaques Infected with SIVmac 251 Randomised into 3 groups according: - peak VL - weight, gender - MHC I Daily Tenofovir and FTC OPAL - (w 4, w 6, w 8, w 10) OPAL TDF + FTC OPAL

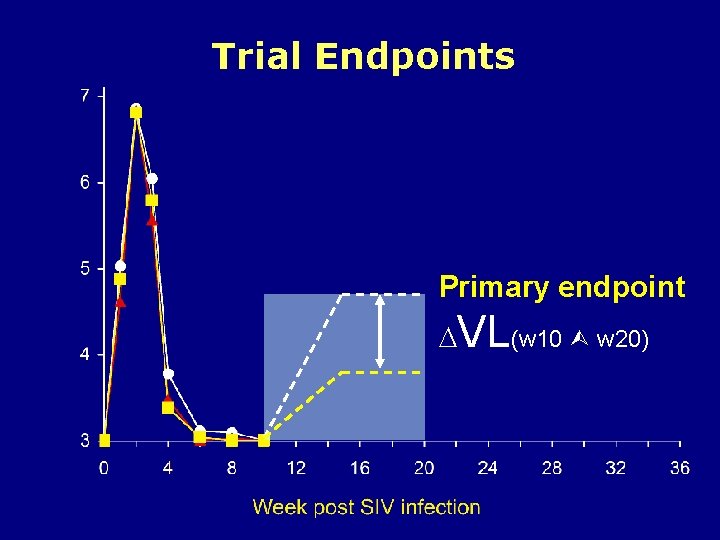
Trial Endpoints Primary endpoint VL(w 10 w 20)

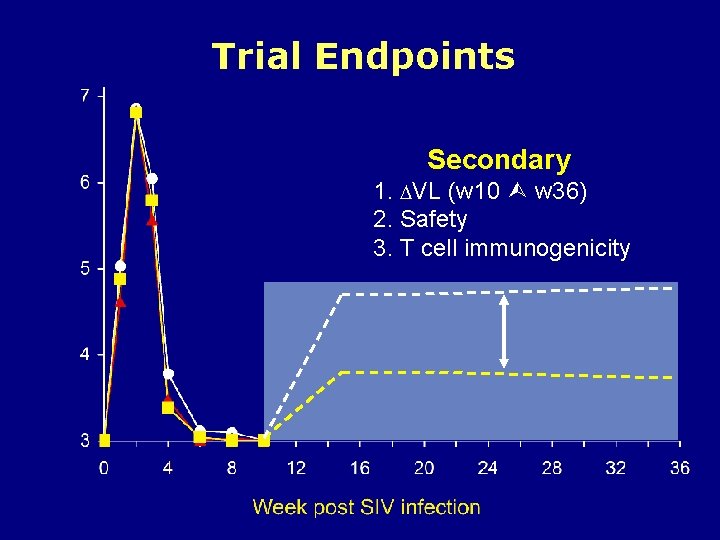
Trial Endpoints Secondary 1. VL (w 10 w 36) 2. Safety 3. T cell immunogenicity

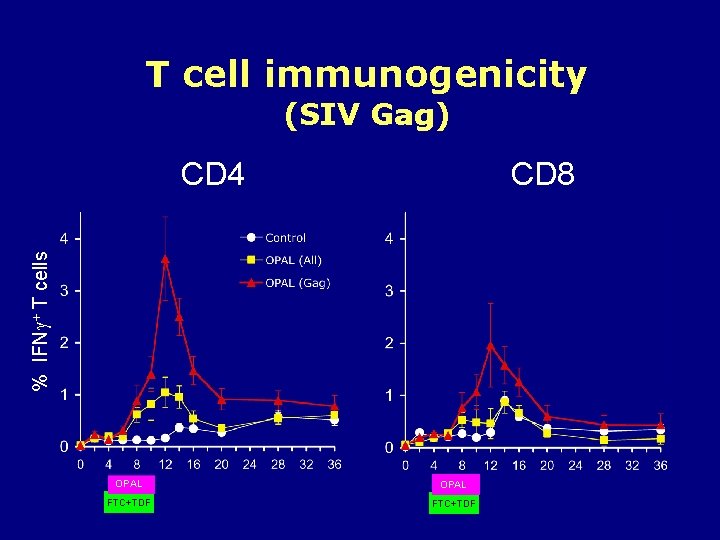
T cell immunogenicity (SIV Gag) CD 8 % IFN + T cells CD 4 OPAL FTC+TDF

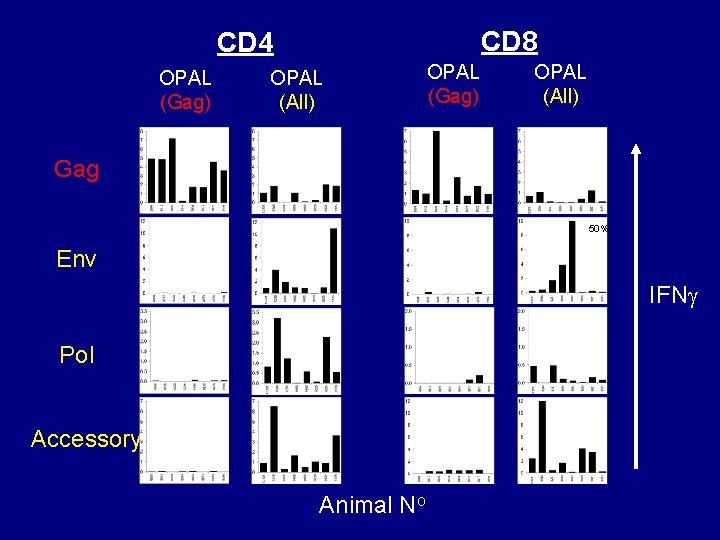
CD 8 CD 4 OPAL (Gag) OPAL (All) Gag 50% Env IFN Pol Accessory Animal No

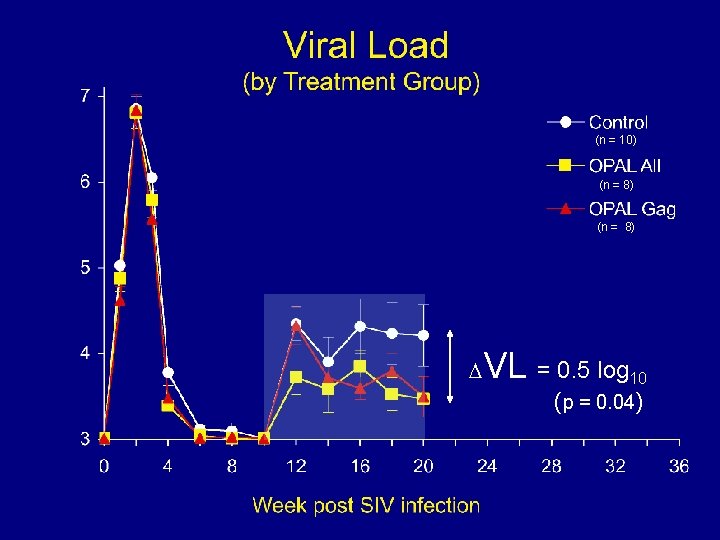
(n = 10) (n = 8) VL = 0. 5 log 10 (p = 0. 04)

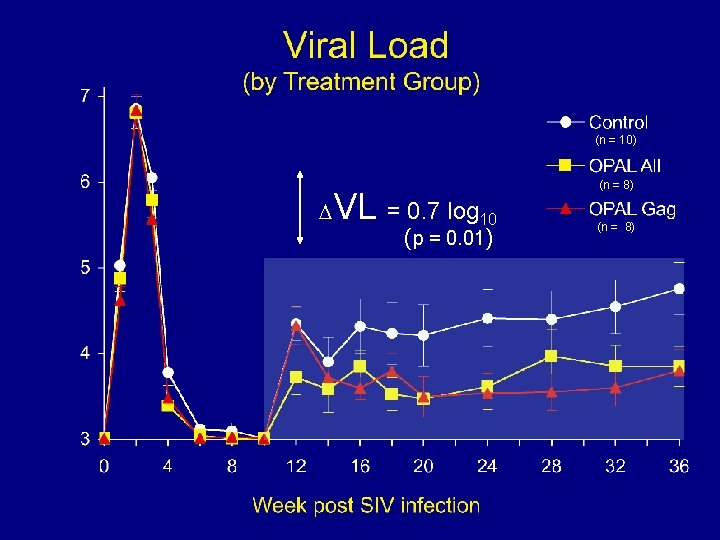
(n = 10) VL = 0. 7 log 10 (p = 0. 01) (n = 8)

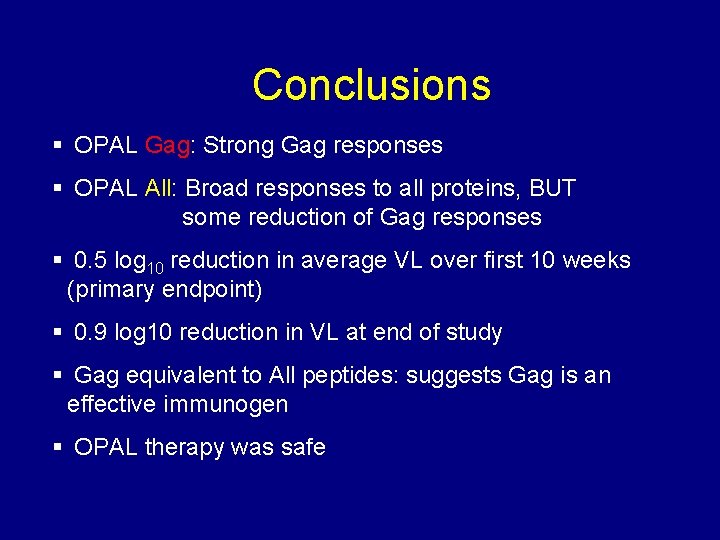
Conclusions § OPAL Gag: Strong Gag responses § OPAL All: Broad responses to all proteins, BUT some reduction of Gag responses § 0. 5 log 10 reduction in average VL over first 10 weeks (primary endpoint) § 0. 9 log 10 reduction in VL at end of study § Gag equivalent to All peptides: suggests Gag is an effective immunogen § OPAL therapy was safe

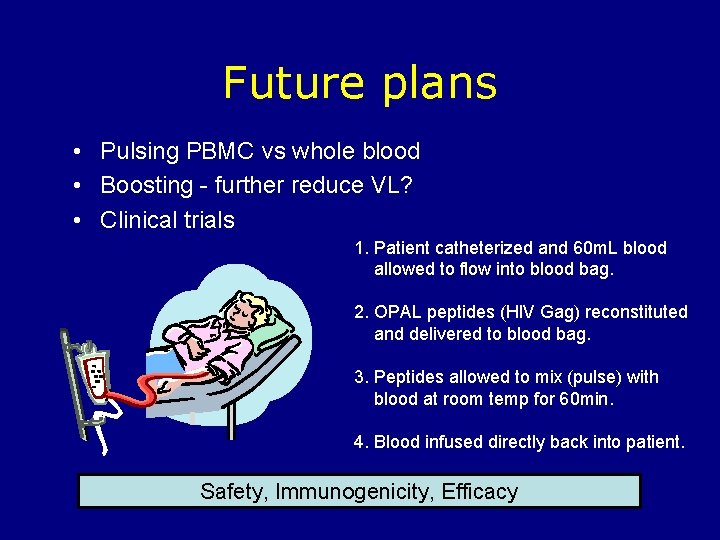
Future plans • Pulsing PBMC vs whole blood • Boosting - further reduce VL? • Clinical trials 1. Patient catheterized and 60 m. L blood allowed to flow into blood bag. 2. OPAL peptides (HIV Gag) reconstituted and delivered to blood bag. 3. Peptides allowed to mix (pulse) with blood at room temp for 60 min. 4. Blood infused directly back into patient. Safety, Immunogenicity, Efficacy

Acknowledgments Stephen Kent Lab (University of Melbourne) Collaborators Matthew Law (UNSW) Stephen Kent Sheila. Jen Alcantara Miranda Smith Caroline Fernandez Viv Peut C. Jane Batten Erik Rollman Liyen Loh Rosemarie Mason Janette Reece Roberta Goli Amanda Handley (OPAL Therapeutics) NIH Reference and Reagent Program Peptides
 Immunotherapy
Immunotherapy Ukons immunotherapy guidelines
Ukons immunotherapy guidelines Rds mcas
Rds mcas Hiv siv
Hiv siv Microelementi
Microelementi Hiv siv
Hiv siv Cnesm siv
Cnesm siv Siv regeln
Siv regeln Peptide identification
Peptide identification Peptide bond importance
Peptide bond importance Peptide hormone receptors
Peptide hormone receptors Peptide bonds in primary structure of protein
Peptide bonds in primary structure of protein Peptide bond dehydration synthesis
Peptide bond dehydration synthesis Cardiac distension
Cardiac distension Diabetes hereditary chart
Diabetes hereditary chart Elevated c peptide
Elevated c peptide Primary secondary tertiary quaternary structure of proteins
Primary secondary tertiary quaternary structure of proteins Number of peptide bonds
Number of peptide bonds