Radioactivity Radioactivity l When an atom of an











- Slides: 16

Radioactivity

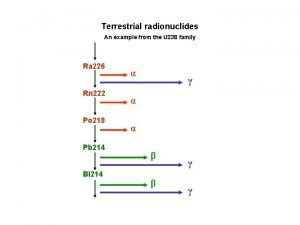
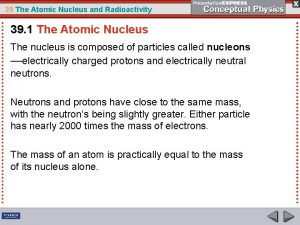
Radioactivity l When an atom of an element has an unstable nucleus, the nucleus can disintegrate, forming subatomic particles and releasing large amounts of energy. l This release is called radioactivity l Alpha and beta particles may be released

Alpha Particles l An alpha particle is a helium nucleus l 2 protons l 2 neutrons l Symbol is α l Mass is 4 amu l Charge is +2

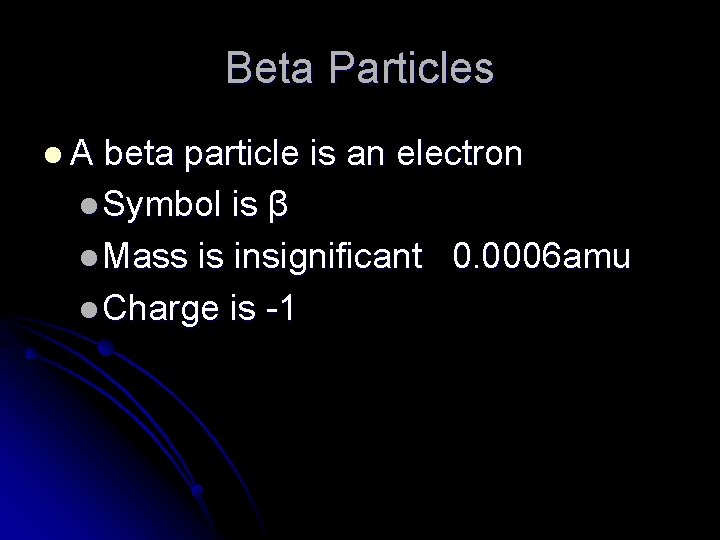
Beta Particles l. A beta particle is an electron l Symbol is β l Mass is insignificant 0. 0006 amu l Charge is -1

Nuclear Reactions During nuclear reactions, an unstable element will transform or decay into another element with a lower mass l In this reaction, an alpha particle has been released l This is called an alpha decay reaction l

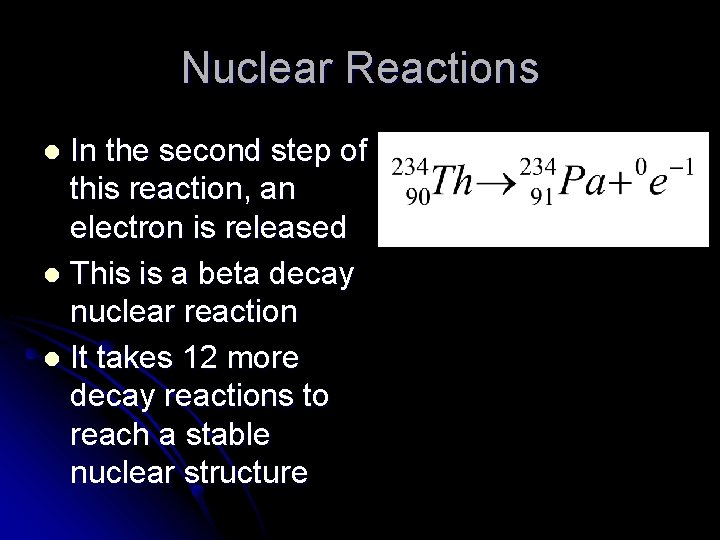
Nuclear Reactions In the second step of this reaction, an electron is released l This is a beta decay nuclear reaction l It takes 12 more decay reactions to reach a stable nuclear structure l

Gamma Rays l Gamma rays are powerful electromagnetic waves that are emitted when a nucleus disintegrates l They do not transform an unstable element into another element or alter its mass

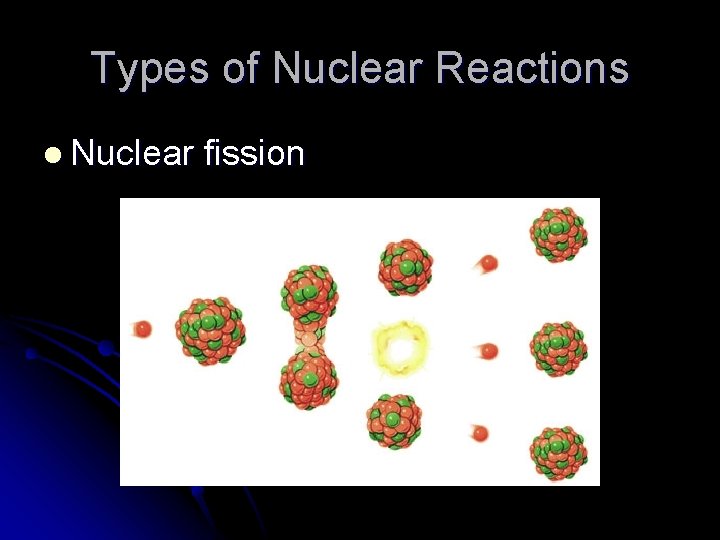
Types of Nuclear Reactions l Nuclear fission l The splitting of a nucleus into two smaller nuclei with a tremendous release of energy l The energy comes from the mass lost when the nucleus is split l Often, neutrons are ejected when the nucleus is split

Types of Nuclear Reactions l Nuclear fission

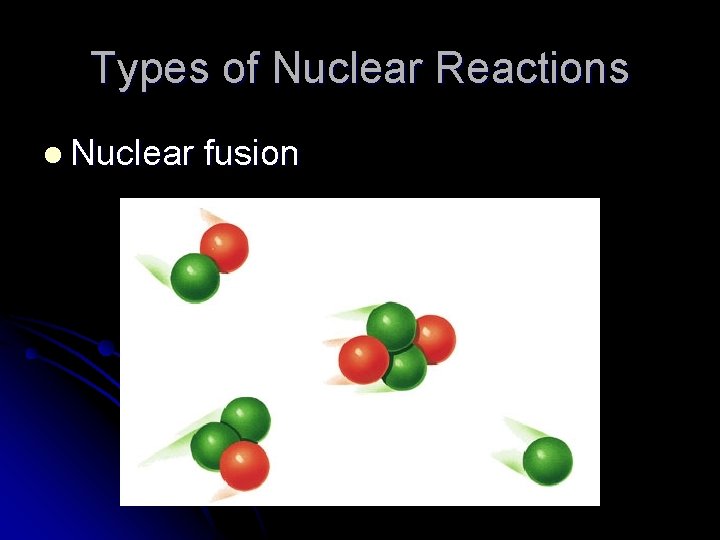
Types of Nuclear Reactions l Nuclear fusion l The combining of two smaller nuclei to form a larger single nucleus with a tremendous release of energy – this energy also comes from lost mass l Fusion requires a huge amount of energy and pressure l Occurs naturally in stars like the sun

Types of Nuclear Reactions l Nuclear fusion

Pros of Nuclear Technology l Used in medicine, weapons and electricity production l Medicine – cancer treatments and diagnostic tests l Electricity – nuclear power plants l. Produce large amounts of energy l. Little air pollutio

Cons of Nuclear Technology l Cooling of electrical plants can result in thermal pollution l Radioactive leaks possible l Fukushima, Japan l Three Mile Island l Dangerous waste must be stored for long periods of time

Half-life l Radioactive materials decay in a predictable way l The rate of decay is called the halflife of a substance l The half-life is the amount of time it takes half of the substance to decay l Different substances have different half-lives

Half-life l For carbon-14 (C-14), the half life is 5370 years l If you start with 1000 g of C-14, in 5370 years, you will have only 500 g left (half has decayed) l After another 5370 years, you will have 250 g (half of the 500 g)

Half-life Activity l We are going to use Skittles to simulate what happens with atoms of radioactive elements
 Kelemahan teori rutherford
Kelemahan teori rutherford The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Becquerel discovery of radioactivity
Becquerel discovery of radioactivity Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Natural vs artificial radioactivity
Natural vs artificial radioactivity Defination of radioactivity
Defination of radioactivity Environmental radioactivity
Environmental radioactivity Radioactivity definition geology
Radioactivity definition geology Who discovered radioactivity
Who discovered radioactivity Units of radioactivity
Units of radioactivity Radioactivity as spontaneous disintegration
Radioactivity as spontaneous disintegration Natural and artificial radioactivity
Natural and artificial radioactivity Defination of radioactivity
Defination of radioactivity Natural radioactivity
Natural radioactivity Atoms and radioactivity
Atoms and radioactivity Who discovered uranium
Who discovered uranium Radioactive formula
Radioactive formula