Quantum Chemistry Lecture No 4 Schrdinger wave equation



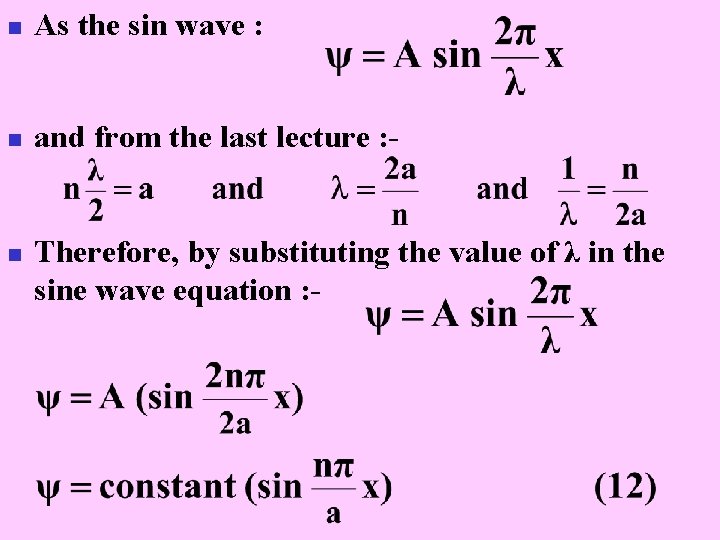
- Slides: 13

Quantum Chemistry Lecture No. 4 Schrödinger wave equation in one dimension Prof. Dr. Samih A. Halawy

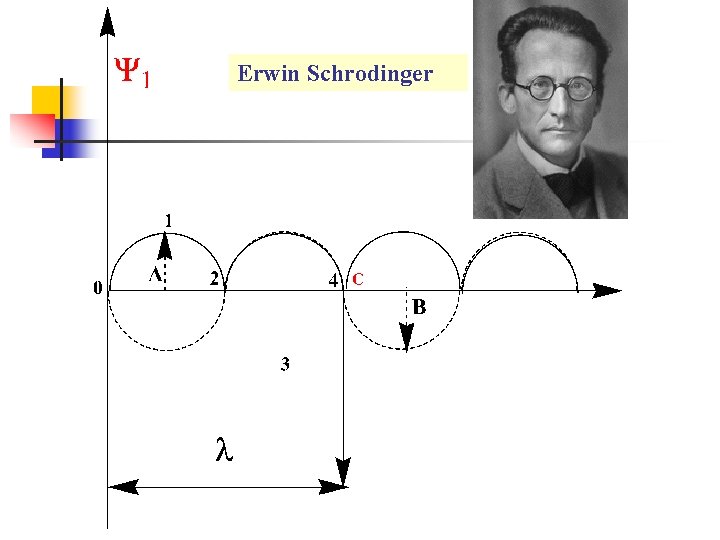
Quantum Chemistry -L 4 Schrödinger wave equation in one dimension : Consider a beam of particles on-a-line segment. These particles have a wave property. Using the (sin wave) to describe the wave movement and the wave function is ( ). The highest point of the wave A, is the amplitude of the wave at various (x) values. n

Erwin Schrodinger

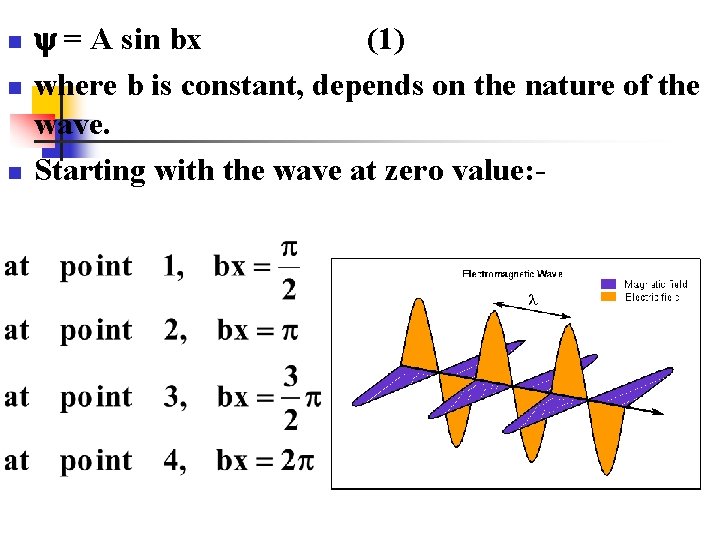
n n n = A sin bx (1) where b is constant, depends on the nature of the wave. Starting with the wave at zero value: -

n n n from zero to point C (4), that gives one wave length = λ, the wave length = x. Equation (1) can be rewritten as : By the same way we can describe the wave function of these particles by (cosine wave): -

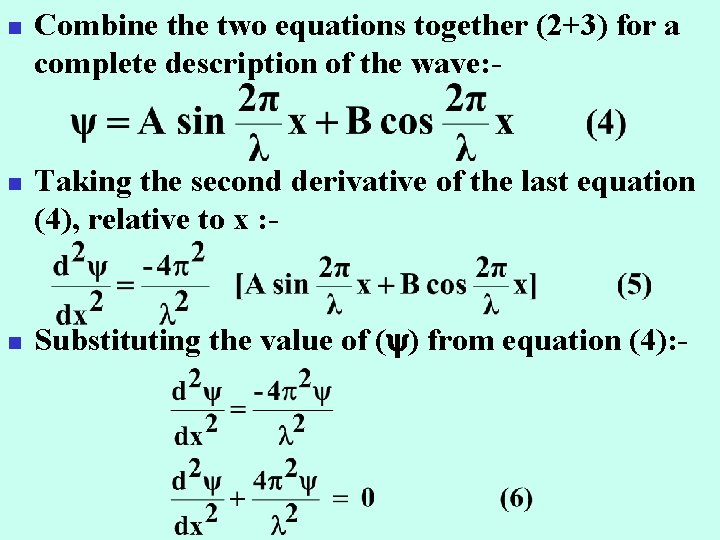
n n n Combine the two equations together (2+3) for a complete description of the wave: - Taking the second derivative of the last equation (4), relative to x : - Substituting the value of ( ) from equation (4): -

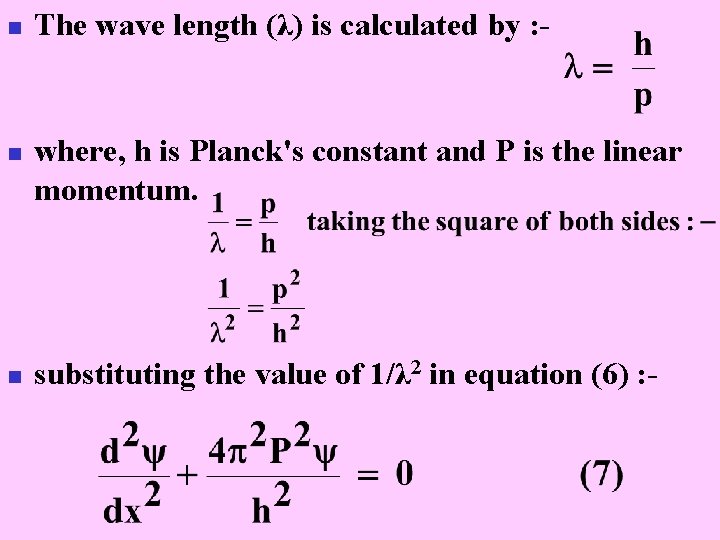
n n n The wave length (λ) is calculated by : - where, h is Planck's constant and P is the linear momentum. substituting the value of 1/λ 2 in equation (6) : -

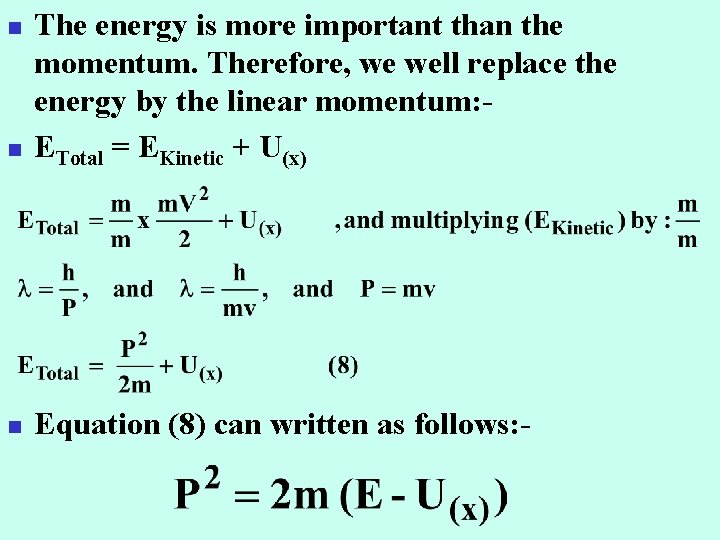
n The energy is more important than the momentum. Therefore, we well replace the energy by the linear momentum: ETotal = EKinetic + U(x) n Equation (8) can written as follows: - n

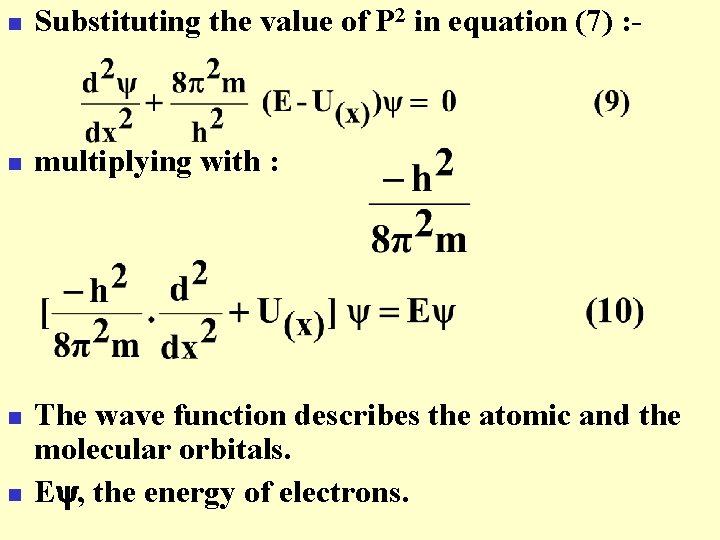
n Substituting the value of P 2 in equation (7) : - n multiplying with : n n The wave function describes the atomic and the molecular orbitals. E , the energy of electrons.

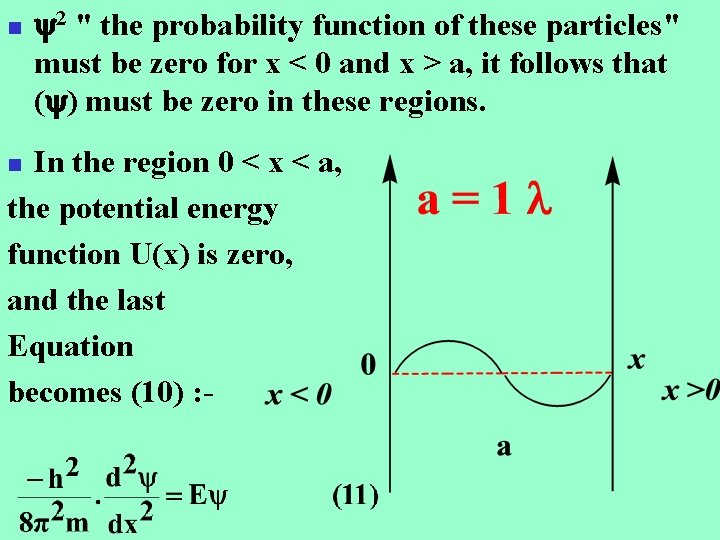
n 2 " the probability function of these particles" must be zero for x < 0 and x > a, it follows that ( ) must be zero in these regions. In the region 0 < x < a, the potential energy function U(x) is zero, and the last Equation becomes (10) : n
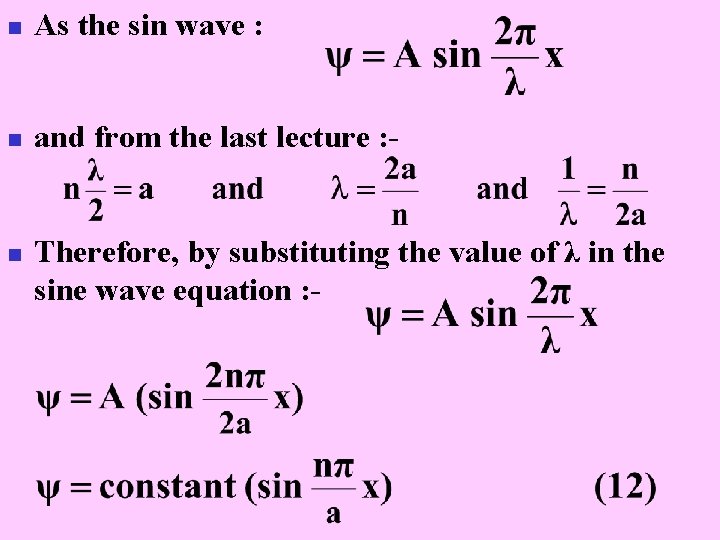
n As the sin wave : n and from the last lecture : - n Therefore, by substituting the value of λ in the sine wave equation : -

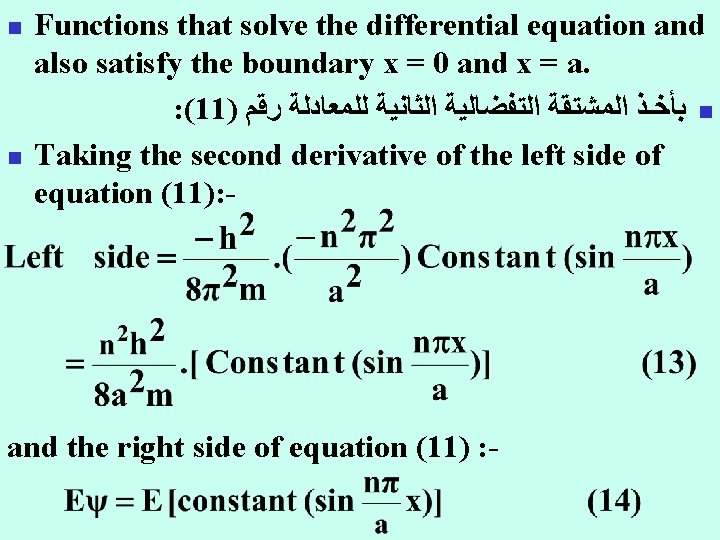
n n Functions that solve the differential equation and also satisfy the boundary x = 0 and x = a. : (11) ﺑﺄﺨـﺬ ﺍﻟﻤﺸﺘﻘﺔ ﺍﻟﺘﻔﻀﺎﻟﻴﺔ ﺍﻟﺜﺎﻧﻴﺔ ﻟﻠﻤﻌﺎﺩﻟﺔ ﺭﻗﻢ n Taking the second derivative of the left side of equation (11): - and the right side of equation (11) : -

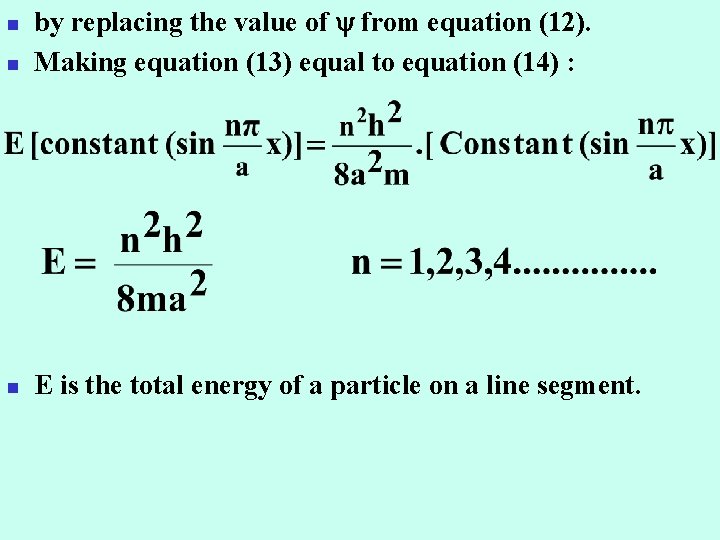
n by replacing the value of from equation (12). Making equation (13) equal to equation (14) : n E is the total energy of a particle on a line segment. n