PTRT 2323 Natural Gas Production Chapter 6 Misc


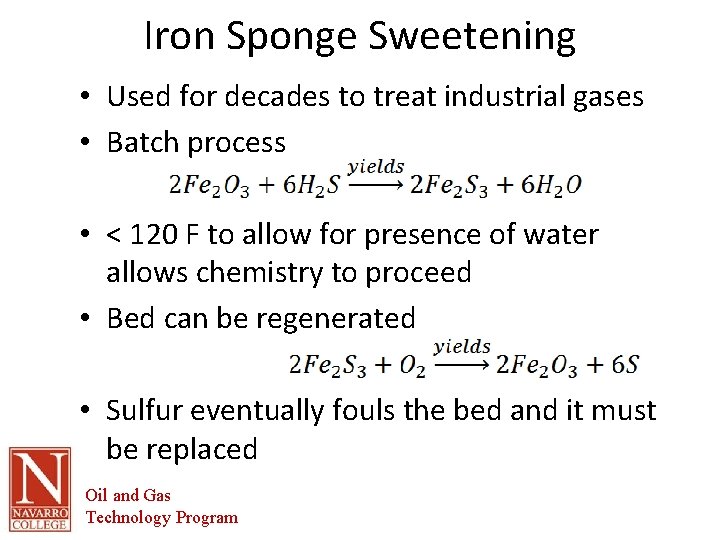



- Slides: 17

PTRT 2323 Natural Gas Production Chapter 6 Misc. Gas Conditioning Oil and Gas Technology Program

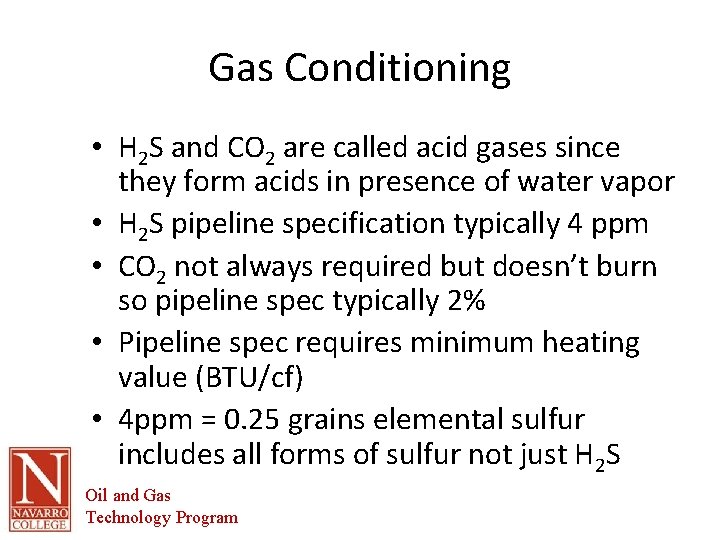
Gas Conditioning • H 2 S and CO 2 are called acid gases since they form acids in presence of water vapor • H 2 S pipeline specification typically 4 ppm • CO 2 not always required but doesn’t burn so pipeline spec typically 2% • Pipeline spec requires minimum heating value (BTU/cf) • 4 ppm = 0. 25 grains elemental sulfur includes all forms of sulfur not just H 2 S Oil and Gas Technology Program

Removing Acid Gases • Iron sponge – oldest but most limited technique – Fe 2 O 3 impregnated wood chips or shavings – Regenerates partially using hot air • Alkanolamine Process – Amine Plant – Continuous – Liquid process – Absorption – Thermal regeneration • Biomass reactor Oil and Gas Technology Program
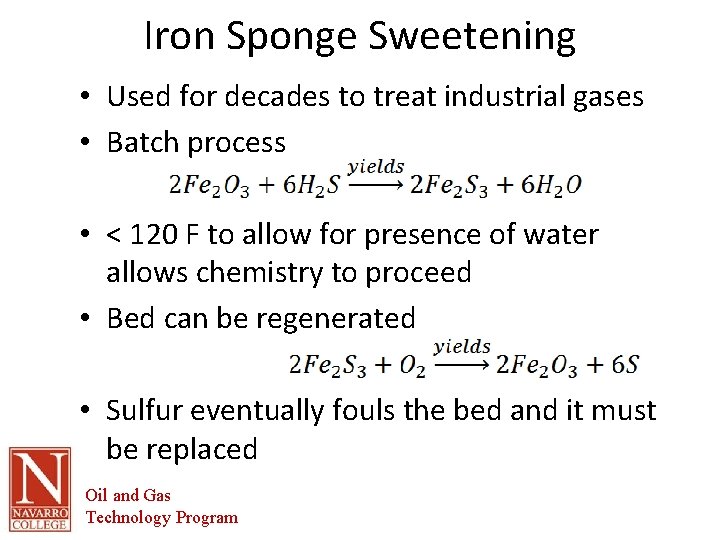
Iron Sponge Sweetening • Used for decades to treat industrial gases • Batch process • < 120 F to allow for presence of water allows chemistry to proceed • Bed can be regenerated • Sulfur eventually fouls the bed and it must be replaced Oil and Gas Technology Program

Iron Sponge Sweetening • Applicable for: – Small gas volumes – Low H 2 S content – Selective for H 2 S – No CO 2 removal • Primary disadvantage is change-out of the bed Oil and Gas Technology Program

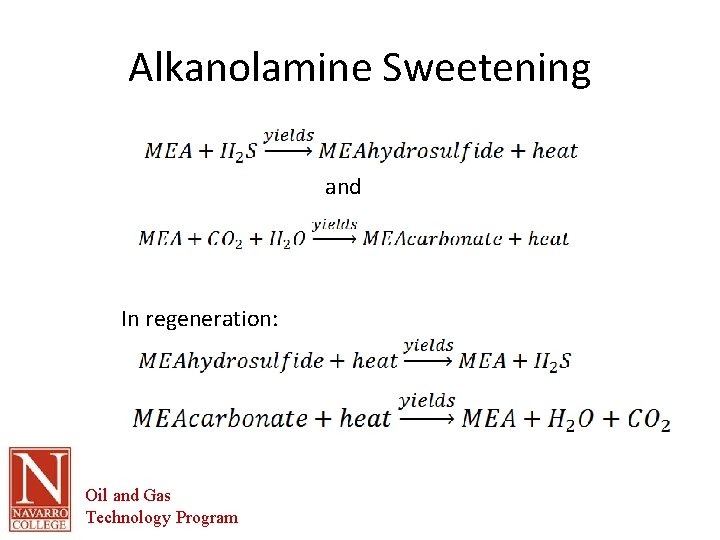
Alkanolamine Sweetening • Alkanolamine – Monoethanolamine (MEA) – Diethanolamine (DEA) – Triethanolamine (TEA) • Non-selective – remove BOTH H 2 S and CO 2 • Weak acid (H 2 S and CO 2) reacts with weak base (MEA, DEA or TEA) to produce a soluble salt Oil and Gas Technology Program

Alkanolamine Sweetening and In regeneration: Oil and Gas Technology Program

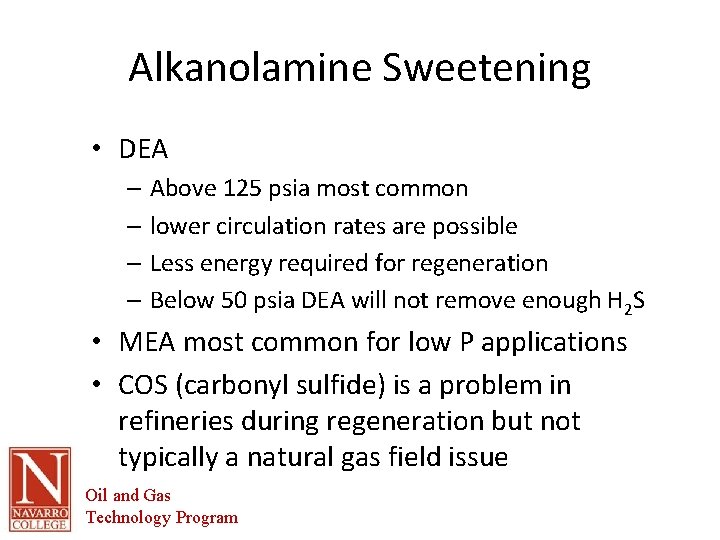
Alkanolamine Sweetening • DEA – Above 125 psia most common – lower circulation rates are possible – Less energy required for regeneration – Below 50 psia DEA will not remove enough H 2 S • MEA most common for low P applications • COS (carbonyl sulfide) is a problem in refineries during regeneration but not typically a natural gas field issue Oil and Gas Technology Program

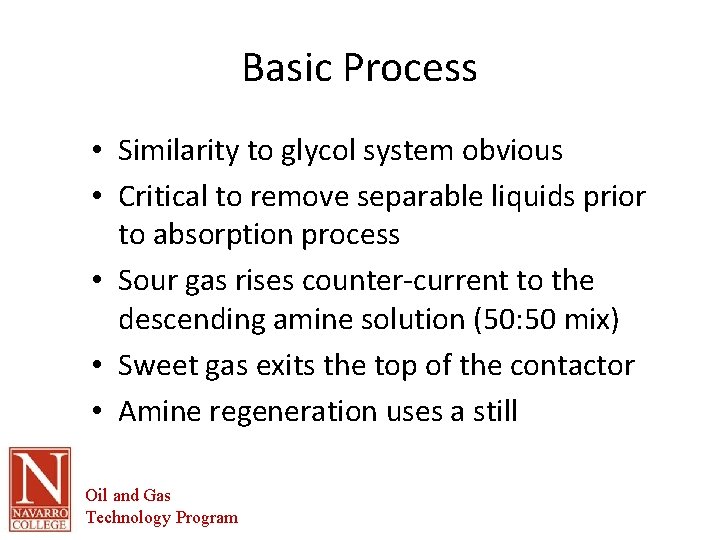
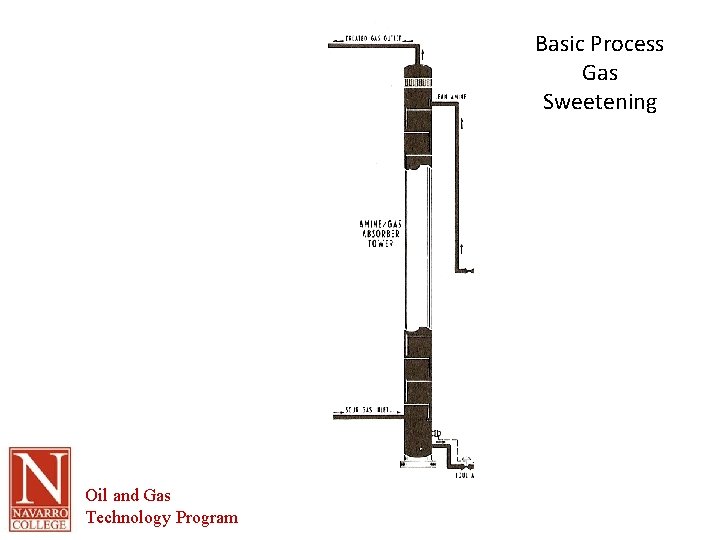
Basic Process • Similarity to glycol system obvious • Critical to remove separable liquids prior to absorption process • Sour gas rises counter-current to the descending amine solution (50: 50 mix) • Sweet gas exits the top of the contactor • Amine regeneration uses a still Oil and Gas Technology Program

Basic Process Gas Sweetening Oil and Gas Technology Program

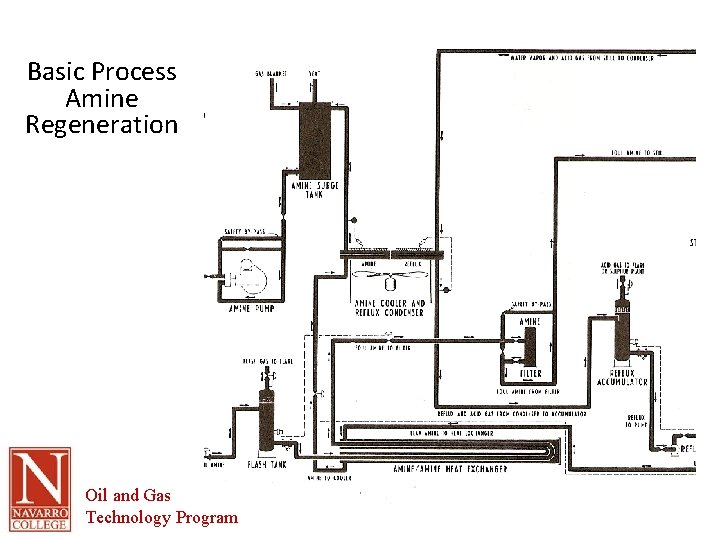
Basic Process Amine Regeneration Oil and Gas Technology Program

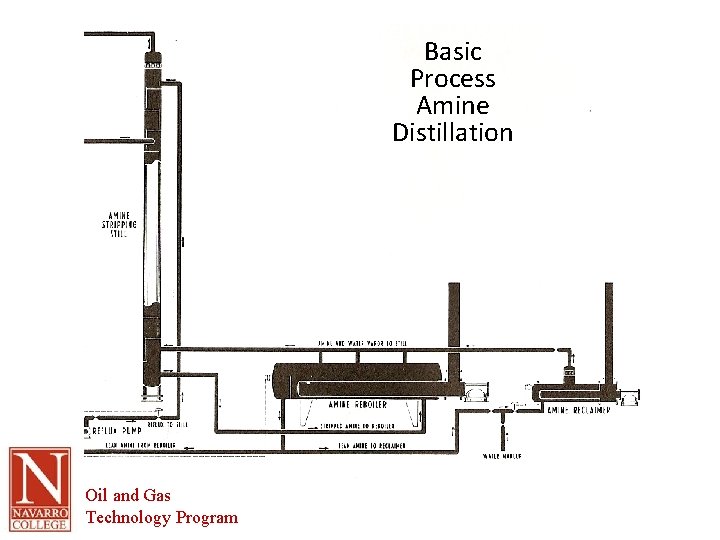
Basic Process Amine Distillation Oil and Gas Technology Program

Amine Regeneration • Rich amine flash tank – Absorbed gases removed by pressure drop – Evolved gases are flared (SO 2) • Heat exchanger T> 190 F • Regeneration still heated by steam rising counter-current to rich amine • Heat shifts equilibrium and liberates H 2 S and CO 2 from the amine solution • Final cooling of the now lean amine done with cooling tower Oil and Gas Technology Program

Glycol/Amine Process • Combines dehydration and sweetening with mixture of treatment chemicals – 10 -30% MEA – 45 -85% Glycol – 5 -25% water • Removes water, H 2 S and CO 2 simultaneously • Lower equipment costs Oil and Gas Technology Program

Disadvantages • Extra losses of MEA caused by higher regeneration temperatures required • Reprocessing requires vacuum distillation • Complex corrosion problems that can be plant specific • ONLY for gas streams that do NOT require low dew points Oil and Gas Technology Program

Sulfinol Process • Mixture of solvents (Shell proprietary) • Advantages – – – Low circulation rates Smaller plant size Low heat capacity of solvent (easier to heat) Low utility costs Low degradation rates Low corrosion rates Low foaming Effective on other sulfur compounds (COS, CS 2 and mercaptans) Low vaporization losses Low fouling of heat exchanger Small expansion during freezing • Disadvantages – Absorption of heavier hydrocarbons – Expense of solvent – Proprietary to Shell (royalty payment) Oil and Gas Technology Program

BTU Control • As more ethane and propane are removed from the gas stream BTU control becomes important • Caution must be taken to avoid inert components (CO 2, N 2) or BTU content will be too low. • Limits amount of ethane and propane that can be removed • Mixing low BTU gas with high BTU gas is an option • BTU content is measured with a calorimeter Oil and Gas Technology Program