PTRT 2323 Natural Gas Production Chapter 1 Characteristics














- Slides: 44

PTRT 2323 Natural Gas Production Chapter 1 Characteristics of Natural Gas Oil and Gas Technology Program

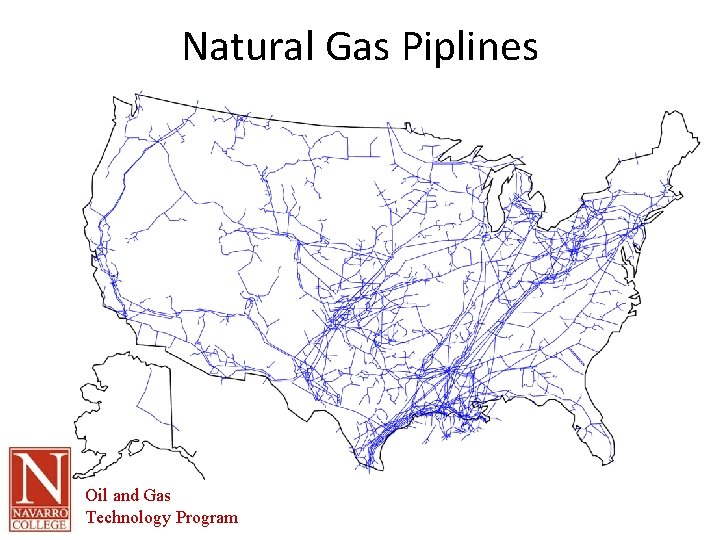
Introduction • Discovery in 1821 in Fredonia, NY and was used locally as a fuel gas in surrounding areas • 1920 – 1930’s a few long pipelines were built (22 -24” diameter and 400 -600 psi) • Huge quantities of gas were flared at well sites due to insufficient markets • Modern natural gas industry began following WWII with 30” diameter carrying gas at 1000 psi • Today virtually all areas of the US are serviced by pipelines Oil and Gas Technology Program

Natural Gas Piplines Oil and Gas Technology Program

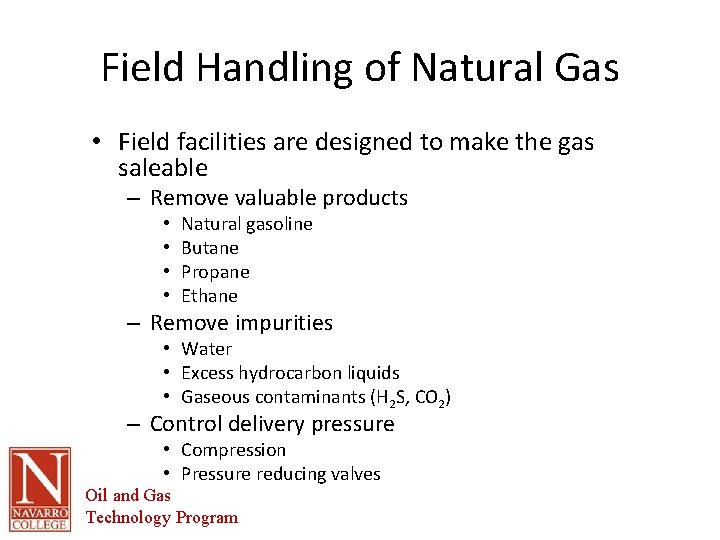
Field Handling of Natural Gas • Field facilities are designed to make the gas saleable – Remove valuable products • • Natural gasoline Butane Propane Ethane – Remove impurities • Water • Excess hydrocarbon liquids • Gaseous contaminants (H 2 S, CO 2) – Control delivery pressure • Compression • Pressure reducing valves Oil and Gas Technology Program

Processing Plants • Remove higher value components – Not needed for sale (excess BTU’s) – More valuable as chemical feed stock or liquid fuel • Remove sulfur compounds and other acid gases • Remove excess water • Subjects covered in summary in this course and in detail in PTRT 1317 and PTRT 1391 Natural Gas Processing I and II Oil and Gas Technology Program

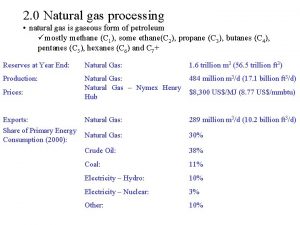
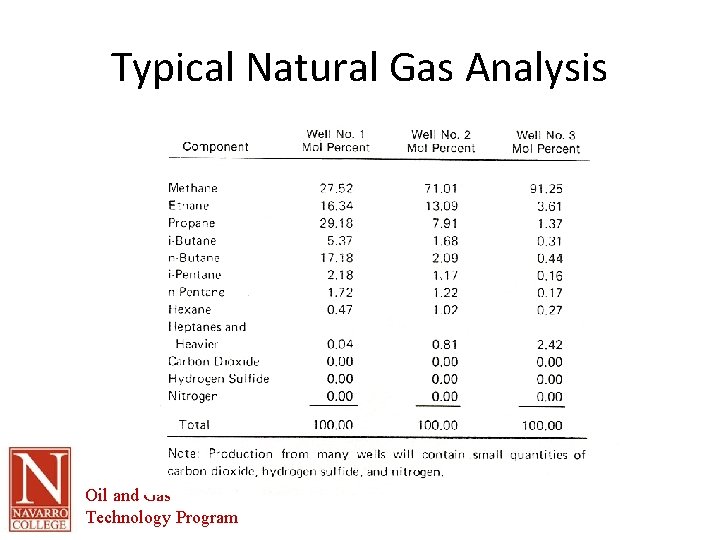
Natural Gas • Mixture of hydrocarbon gases together with some impurities • Impurities – Water vapor – Heavier hydrocarbons – Hydrogen sulfide and other acid gases • Hydrocarbon gases – – – Methane Ethane Propane Butanes Others • Ethane and heavier molecules are removed for additional processing because of their value • What reaches market is typically 95%-98% Methane Oil and Gas Technology Program

Typical Natural Gas Analysis Oil and Gas Technology Program

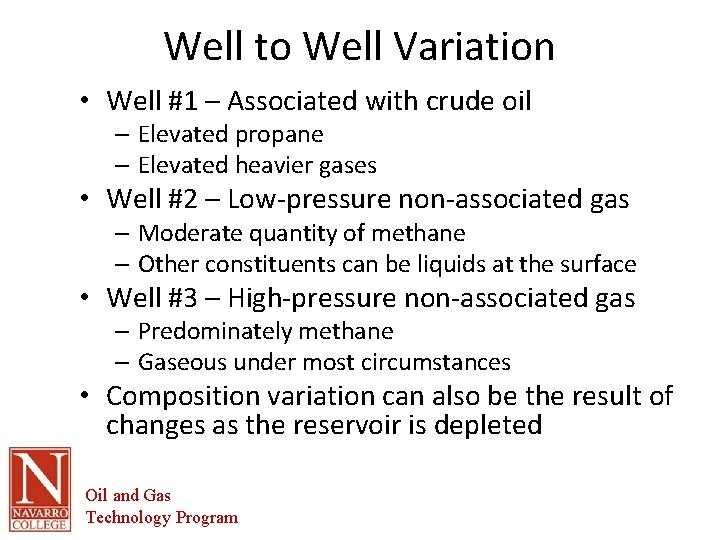
Well to Well Variation • Well #1 – Associated with crude oil – Elevated propane – Elevated heavier gases • Well #2 – Low-pressure non-associated gas – Moderate quantity of methane – Other constituents can be liquids at the surface • Well #3 – High-pressure non-associated gas – Predominately methane – Gaseous under most circumstances • Composition variation can also be the result of changes as the reservoir is depleted Oil and Gas Technology Program

Why does methane tell us about the gas pressure at the surface? ? • At 15 ⁰C – Methane has density ρ = 0. 68 kg/m 3 – Ethane has density ρ = 1. 282 kg/m 3 – Air has density ρ = 1. 202 kg/m 3 • Methane is trying to RISE • Ethane is trying to SINK Oil and Gas Technology Program

Chemical Structures • Natural gas normally composed of a mixture of straight-chain hydrocarbons (paraffins) • Cyclic compounds can also be found in the mixture. • Straight-chain and cyclic refer to the molecular structure Oil and Gas Technology Program

Chemical Structures Isomers Oil and Gas Technology Program

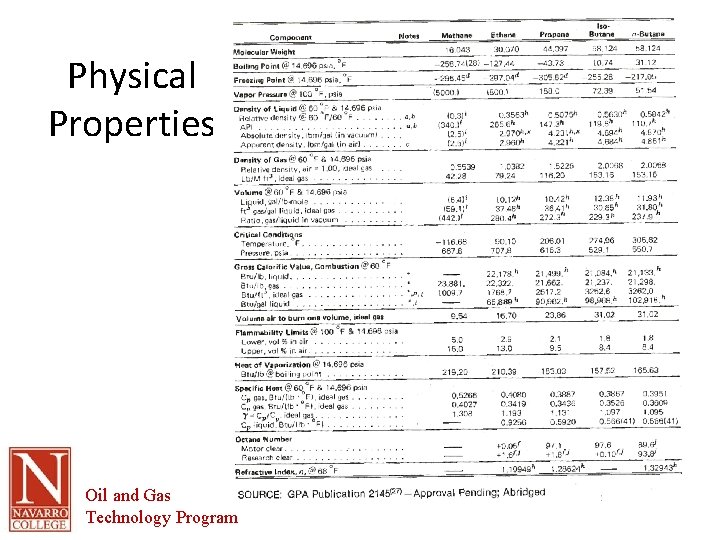
Physical Properties Oil and Gas Technology Program

Physical Properties • Important to know the properties of each individual component of the gas • Properties of the mixture will be determined by combining the properties of each individual component • RULE OF MIXTURES Oil and Gas Technology Program

Physical Properties • Typically important properties are: – Molecular weight – Freezing point – Boiling point – Density – Critical temperature – Critical pressure – Heat of vaporization – Specific heat Oil and Gas Technology Program

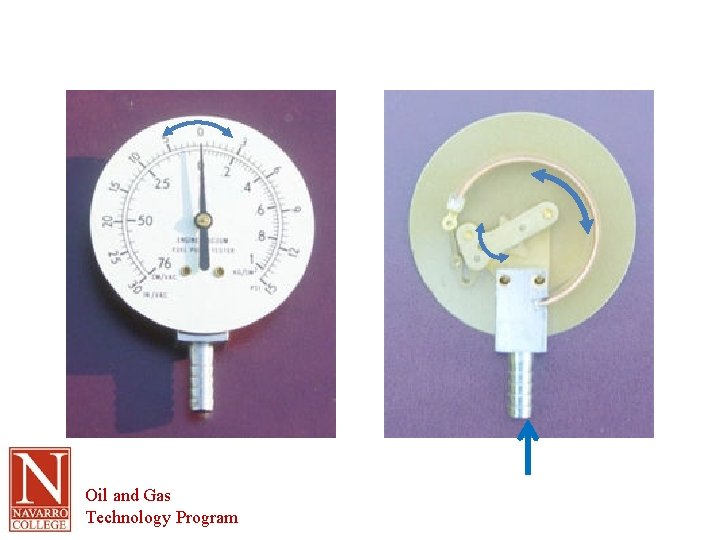
Terminology • Gauge pressure – Pressure indicated above or below atmospheric pressure (14. 7 psia or 29. 92” Hg) – Read on a gauge (usually) – Uses a Bourdon tube for the measurement • Tube flexes as a result of differential pressure • Normally read in psig • On psig scale 1 atmosphere = 0 psig Oil and Gas Technology Program

Oil and Gas Technology Program

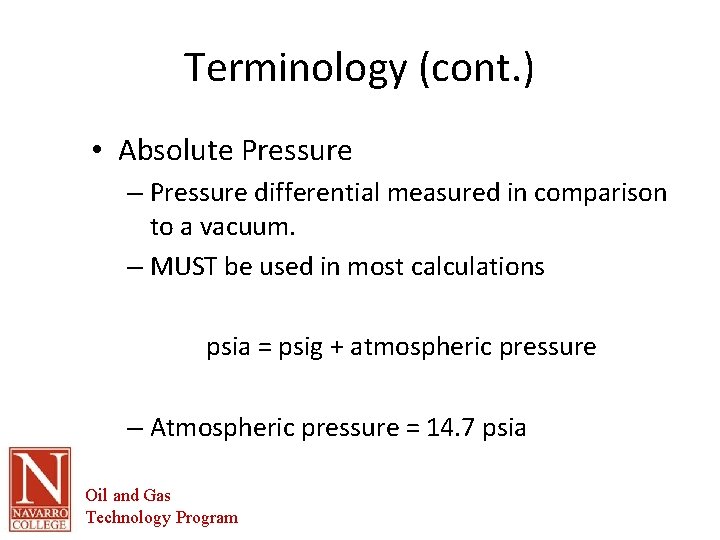
Terminology (cont. ) • Absolute Pressure – Pressure differential measured in comparison to a vacuum. – MUST be used in most calculations psia = psig + atmospheric pressure – Atmospheric pressure = 14. 7 psia Oil and Gas Technology Program

Terminology (cont. ) • Vapor Pressure • Vapor reached equilibrium with the liquid in a CLOSED container – Rate of evaporation = rate of condensation • http: //www. wisconline. com/objects/index_tj. asp? obj. ID=GC H 4304 • Only requires SOME liquid to be present Oil and Gas Technology Program

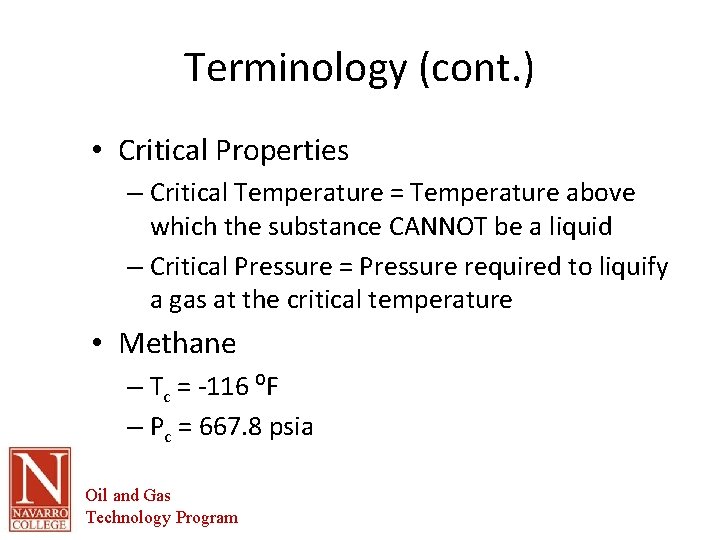
Terminology (cont. ) • Critical Properties – Critical Temperature = Temperature above which the substance CANNOT be a liquid – Critical Pressure = Pressure required to liquify a gas at the critical temperature • Methane – Tc = -116 ⁰F – Pc = 667. 8 psia Oil and Gas Technology Program

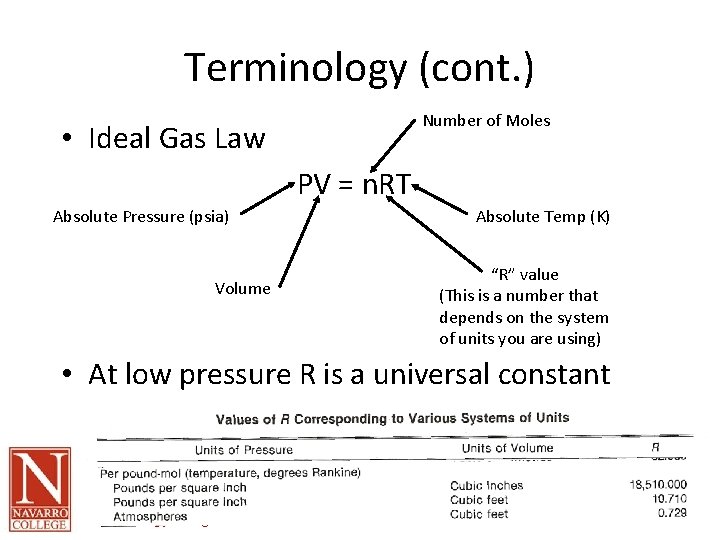
Terminology (cont. ) Number of Moles • Ideal Gas Law PV = n. RT Absolute Pressure (psia) Volume Absolute Temp (K) “R” value (This is a number that depends on the system of units you are using) • At low pressure R is a universal constant Oil and Gas Technology Program

Using the Ideal Gas Law Problem Type A Which reduces to: If you know the starting conditions (1) and can define any two of the final conditions (2) then you can solve for the remaining term Oil and Gas Technology Program

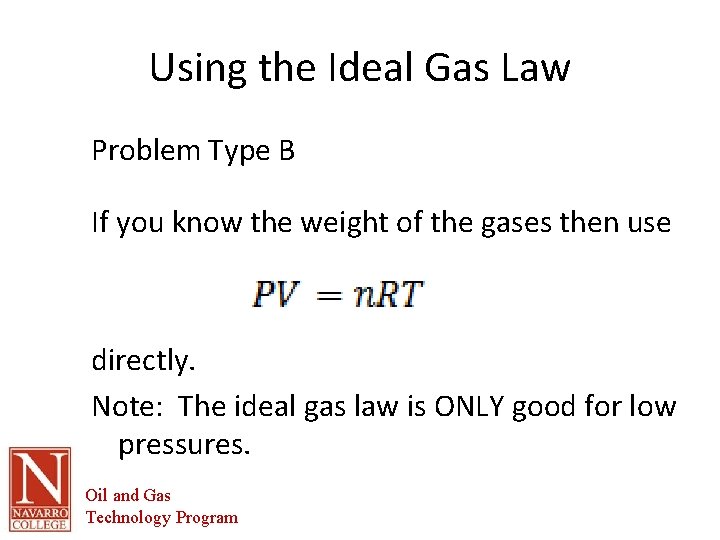
Using the Ideal Gas Law Problem Type B If you know the weight of the gases then use directly. Note: The ideal gas law is ONLY good for low pressures. Oil and Gas Technology Program

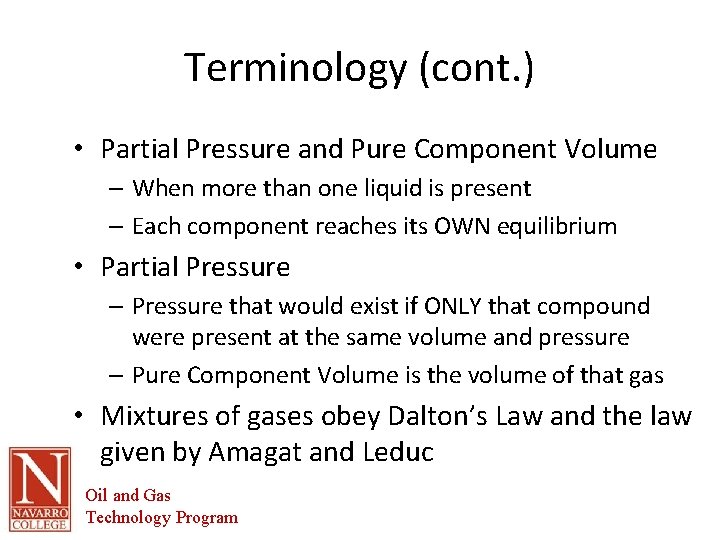
Terminology (cont. ) • Partial Pressure and Pure Component Volume – When more than one liquid is present – Each component reaches its OWN equilibrium • Partial Pressure – Pressure that would exist if ONLY that compound were present at the same volume and pressure – Pure Component Volume is the volume of that gas • Mixtures of gases obey Dalton’s Law and the law given by Amagat and Leduc Oil and Gas Technology Program

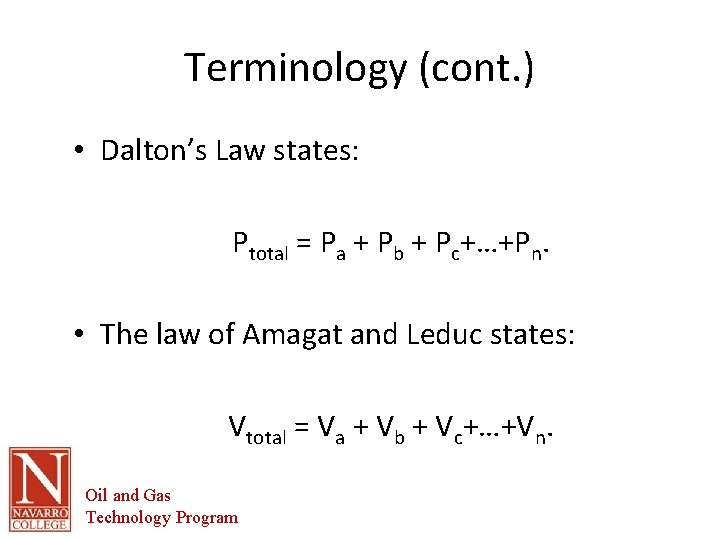
Terminology (cont. ) • Dalton’s Law states: Ptotal = Pa + Pb + Pc+…+Pn. • The law of Amagat and Leduc states: Vtotal = Va + Vb + Vc+…+Vn. Oil and Gas Technology Program

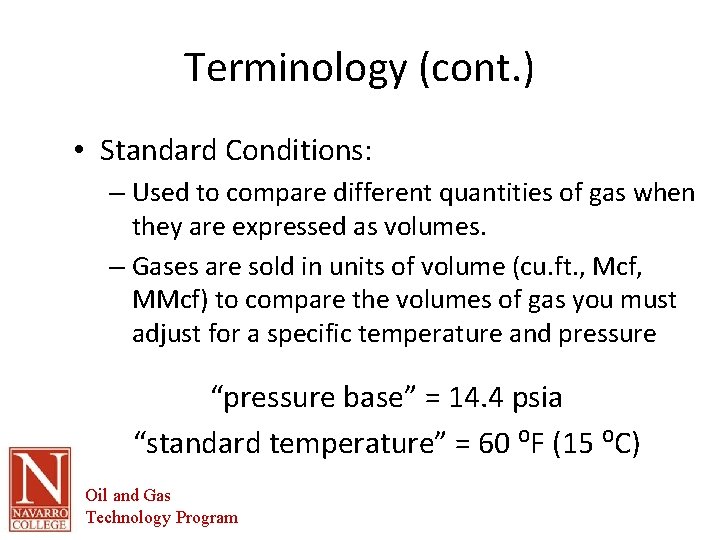
Terminology (cont. ) • Standard Conditions: – Used to compare different quantities of gas when they are expressed as volumes. – Gases are sold in units of volume (cu. ft. , Mcf, MMcf) to compare the volumes of gas you must adjust for a specific temperature and pressure “pressure base” = 14. 4 psia “standard temperature” = 60 ⁰F (15 ⁰C) Oil and Gas Technology Program

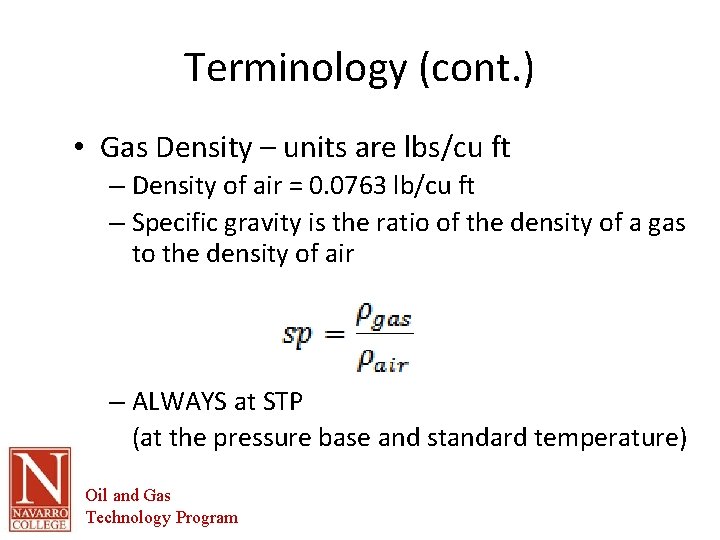
Terminology (cont. ) • Gas Density – units are lbs/cu ft – Density of air = 0. 0763 lb/cu ft – Specific gravity is the ratio of the density of a gas to the density of air – ALWAYS at STP (at the pressure base and standard temperature) Oil and Gas Technology Program

Terminology (cont. ) • Liquid Density – units are lbs/cu ft – Density of water = 62. 43 lb/cu ft (8. 33 lb/gal) – Specific gravity is the ratio of the density of a liquid to the density of water at 60 ⁰F – ALWAYS at 60 ⁰F (standard temperature) • API Gravity – units in degrees Oil and Gas Technology Program

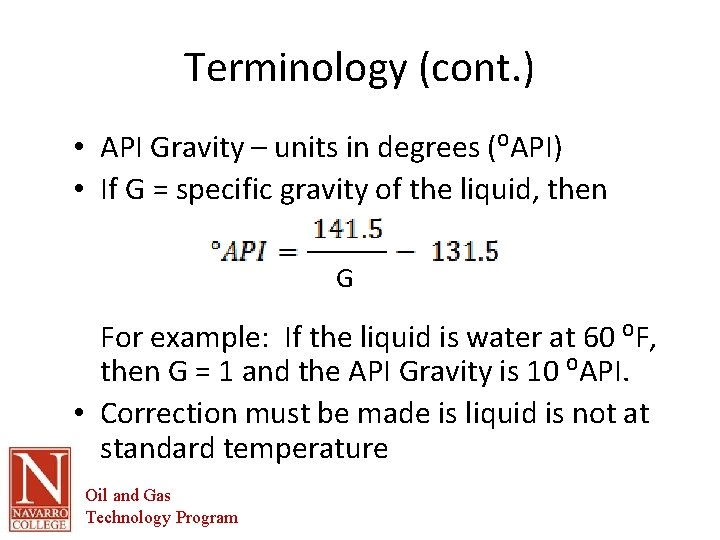
Terminology (cont. ) • API Gravity – units in degrees (⁰API) • If G = specific gravity of the liquid, then G For example: If the liquid is water at 60 ⁰F, then G = 1 and the API Gravity is 10 ⁰API. • Correction must be made is liquid is not at standard temperature Oil and Gas Technology Program

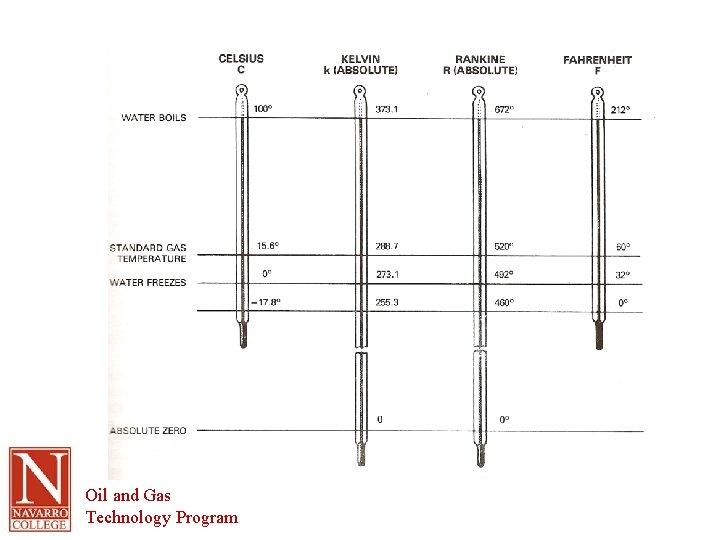
Terminology (cont. ) • Temperature – ⁰F in the US – ⁰C everywhere else (SI unit) • Absolute Temperature – Absolute zero – temperature at which molecular vibration theoretically stops. – Absolute temperature measured in K – 0 K = -273. 1 ⁰C Oil and Gas Technology Program

Oil and Gas Technology Program

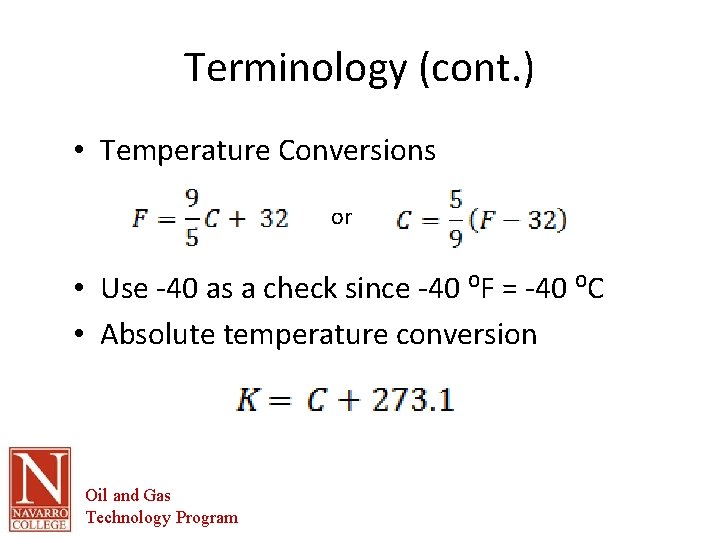
Terminology (cont. ) • Temperature Conversions or • Use -40 as a check since -40 ⁰F = -40 ⁰C • Absolute temperature conversion Oil and Gas Technology Program

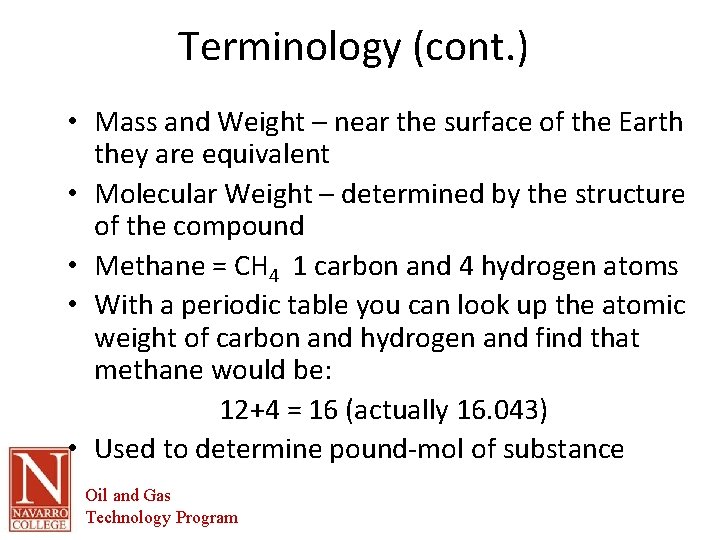
Terminology (cont. ) • Mass and Weight – near the surface of the Earth they are equivalent • Molecular Weight – determined by the structure of the compound • Methane = CH 4 1 carbon and 4 hydrogen atoms • With a periodic table you can look up the atomic weight of carbon and hydrogen and find that methane would be: 12+4 = 16 (actually 16. 043) • Used to determine pound-mol of substance Oil and Gas Technology Program

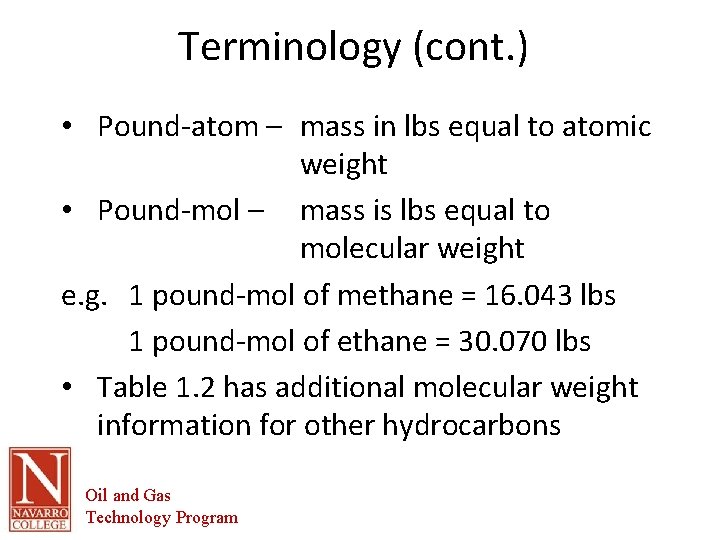
Terminology (cont. ) • Pound-atom – mass in lbs equal to atomic weight • Pound-mol – mass is lbs equal to molecular weight e. g. 1 pound-mol of methane = 16. 043 lbs 1 pound-mol of ethane = 30. 070 lbs • Table 1. 2 has additional molecular weight information for other hydrocarbons Oil and Gas Technology Program

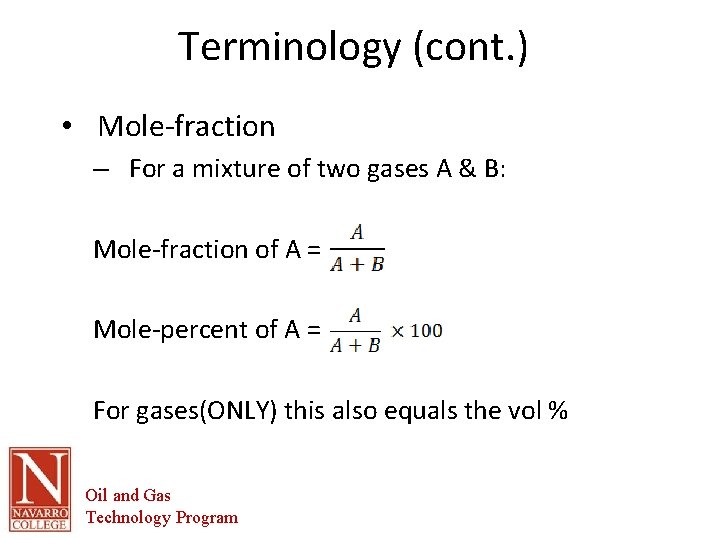
Terminology (cont. ) • Mole-fraction – For a mixture of two gases A & B: Mole-fraction of A = Mole-percent of A = For gases(ONLY) this also equals the vol % Oil and Gas Technology Program

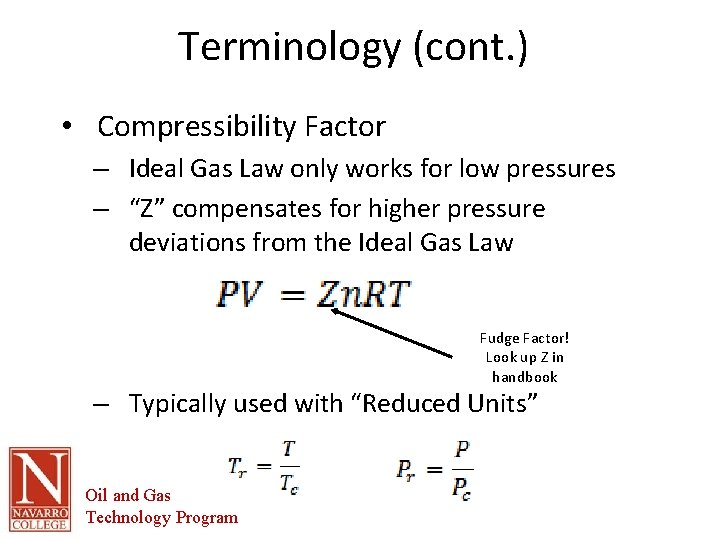
Terminology (cont. ) • Compressibility Factor – Ideal Gas Law only works for low pressures – “Z” compensates for higher pressure deviations from the Ideal Gas Law Fudge Factor! Look up Z in handbook – Typically used with “Reduced Units” Oil and Gas Technology Program

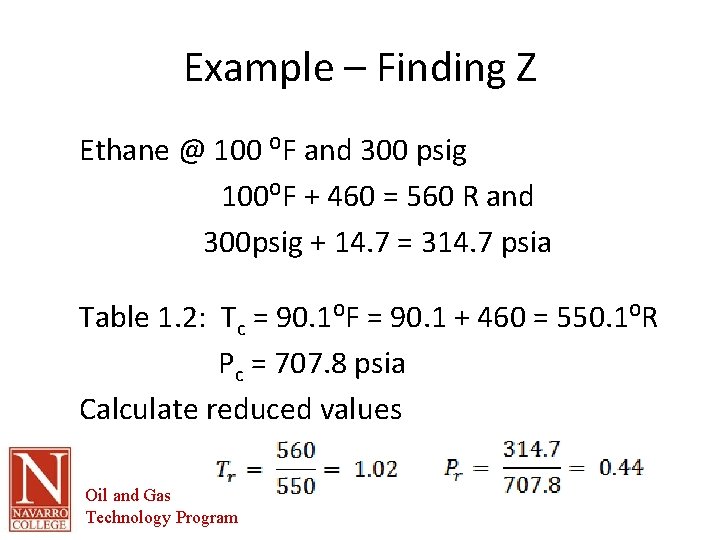
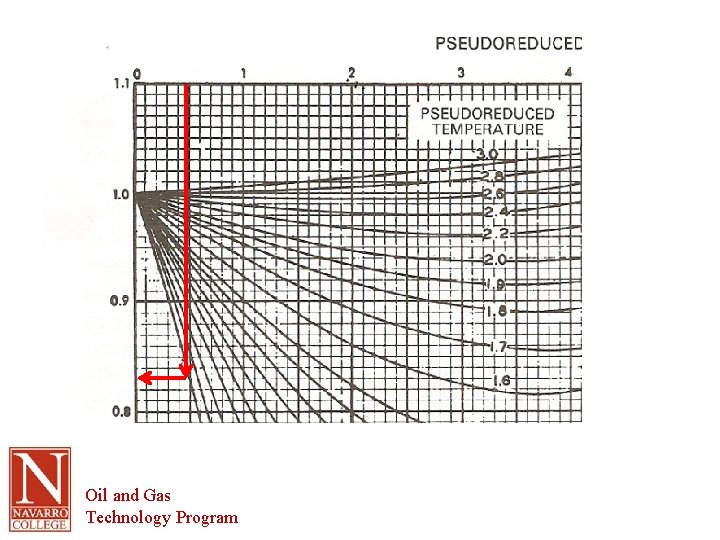
Example – Finding Z Ethane @ 100 ⁰F and 300 psig 100⁰F + 460 = 560 R and 300 psig + 14. 7 = 314. 7 psia Table 1. 2: Tc = 90. 1⁰F = 90. 1 + 460 = 550. 1⁰R Pc = 707. 8 psia Calculate reduced values Oil and Gas Technology Program

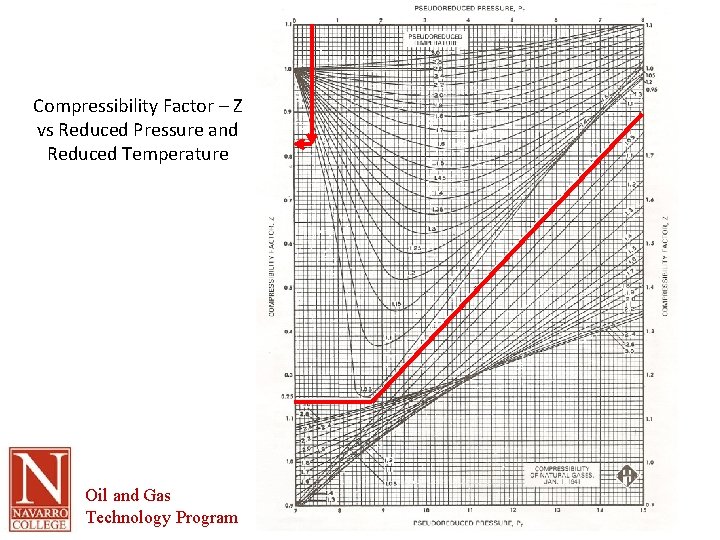
Compressibility Factor – Z vs Reduced Pressure and Reduced Temperature Oil and Gas Technology Program

Oil and Gas Technology Program

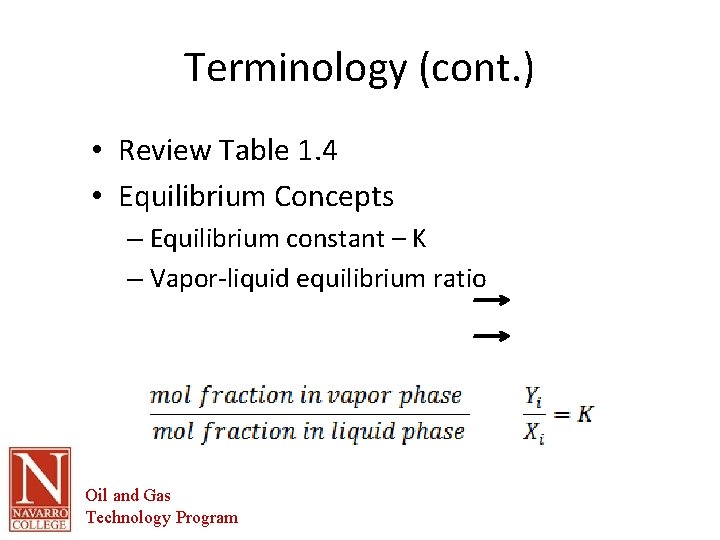
Terminology (cont. ) • Review Table 1. 4 • Equilibrium Concepts – Equilibrium constant – K – Vapor-liquid equilibrium ratio Oil and Gas Technology Program

X X X Oil and Gas Technology Program = = =

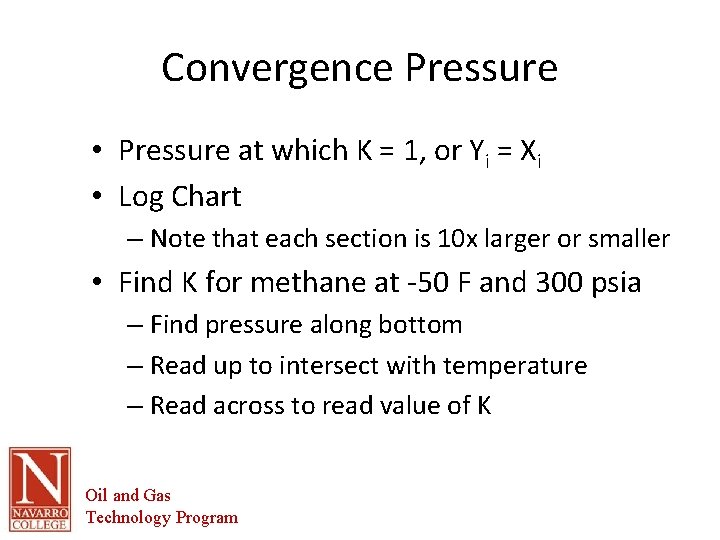
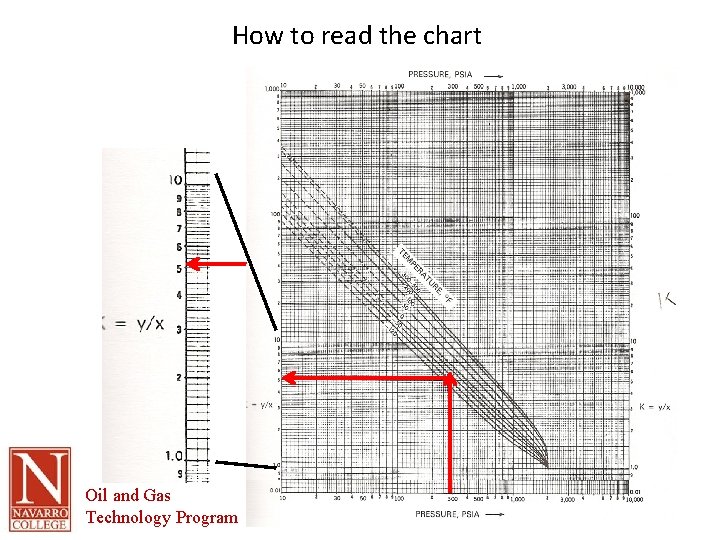
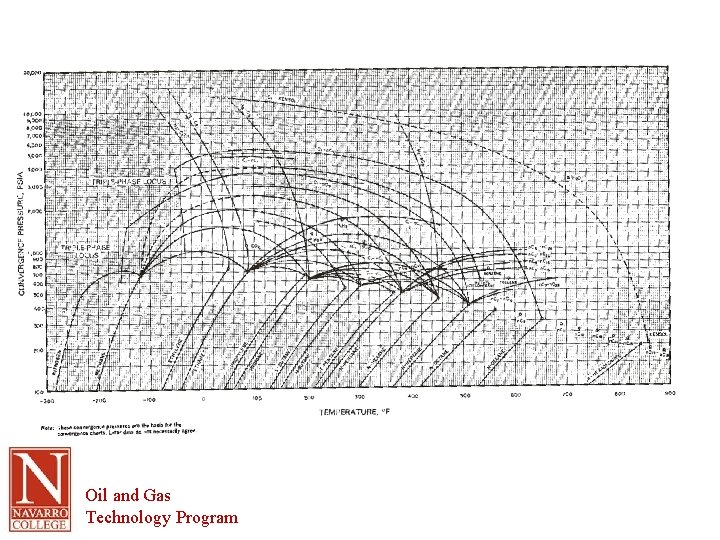
Convergence Pressure • Pressure at which K = 1, or Yi = Xi • Log Chart – Note that each section is 10 x larger or smaller • Find K for methane at -50 F and 300 psia – Find pressure along bottom – Read up to intersect with temperature – Read across to read value of K Oil and Gas Technology Program

How to read the chart Oil and Gas Technology Program

Terminology (cont. ) • Bubble point – temperature at which the first bubble forms at a given pressure – Ki. Ni = 1. 0 at the bubble point • Dew Point – Temperature for the first droplet of liquid to form at a given pressure – Ni/Ki = 1. 0 at the dew point • Table 1. 7 shows convergence pressure Oil and Gas Technology Program

Oil and Gas Technology Program
 The decimal expansion of 2323×52 is
The decimal expansion of 2323×52 is Red nacional de laboratorios
Red nacional de laboratorios Production pre production post production
Production pre production post production Ideal gas vs perfect gas
Ideal gas vs perfect gas An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Reason for bhopal gas tragedy
Reason for bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Volume molare
Volume molare Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Reaksi pembentukan gas no2f dari gas no2 dan f2
Reaksi pembentukan gas no2f dari gas no2 dan f2 Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Electricity pros and cons
Electricity pros and cons Portal de habilitaciones de gas natural osinergmin
Portal de habilitaciones de gas natural osinergmin Advantages of petroleum
Advantages of petroleum Greenhouse impact
Greenhouse impact How natural gas is formed
How natural gas is formed Advantages of geothermal energy
Advantages of geothermal energy Odorometer natural gas
Odorometer natural gas Gas natural fenosa
Gas natural fenosa Up gnv consumo
Up gnv consumo Natral gas
Natral gas Line vane separator
Line vane separator Fracking natural gas
Fracking natural gas Contour strip mining
Contour strip mining Offshore drilling
Offshore drilling Información sobre el gas natural
Información sobre el gas natural Petroleum and natural gas formation
Petroleum and natural gas formation Natural hazards vs natural disasters
Natural hazards vs natural disasters Natural income
Natural income Explain production system characteristics
Explain production system characteristics Characteristics of agricultural production
Characteristics of agricultural production Noble gases properties
Noble gases properties Characteristics of ideal gas
Characteristics of ideal gas Natural church development 8 characteristics
Natural church development 8 characteristics Finely tuned input
Finely tuned input Natural church development 8 characteristics
Natural church development 8 characteristics Chapter 5 section 2 costs of production
Chapter 5 section 2 costs of production Chapter 13 study guide production and business operations
Chapter 13 study guide production and business operations Chapter 25 production and growth
Chapter 25 production and growth Chapter 18 the markets for the factors of production
Chapter 18 the markets for the factors of production Mankiw chapter 13
Mankiw chapter 13