Polymers PART 2 Soft Condensed Matter Physics Dept





- Slides: 18

Polymers PART. 2 Soft Condensed Matter Physics Dept. Phys. , Tunghai Univ. C. T. Shih

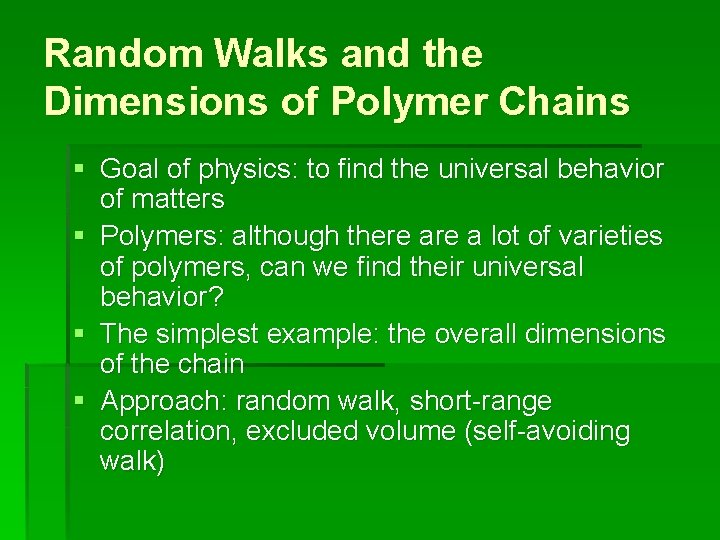
Random Walks and the Dimensions of Polymer Chains § Goal of physics: to find the universal behavior of matters § Polymers: although there a lot of varieties of polymers, can we find their universal behavior? § The simplest example: the overall dimensions of the chain § Approach: random walk, short-range correlation, excluded volume (self-avoiding walk)

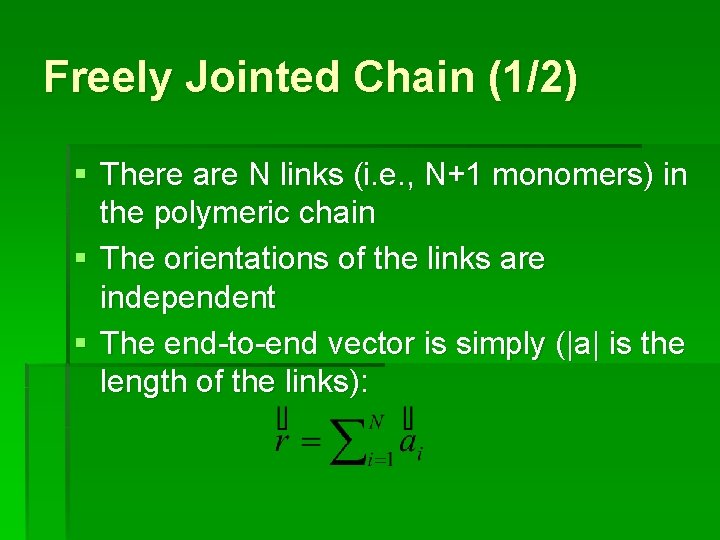
Freely Jointed Chain (1/2) § There are N links (i. e. , N+1 monomers) in the polymeric chain § The orientations of the links are independent § The end-to-end vector is simply (|a| is the length of the links):

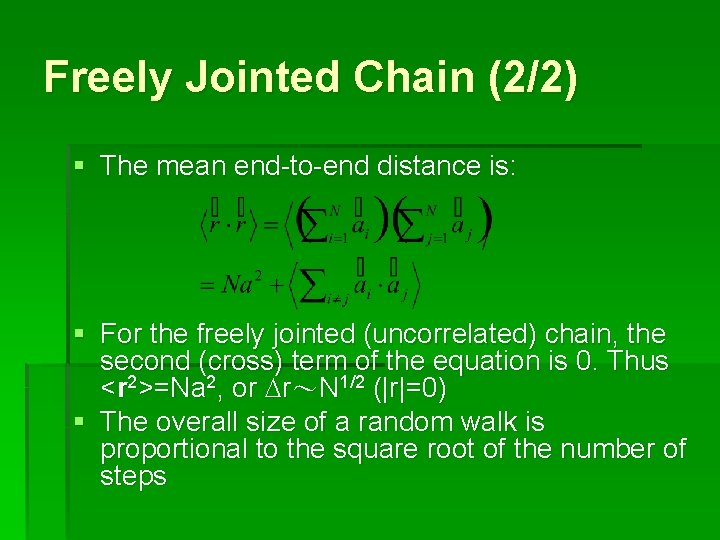
Freely Jointed Chain (2/2) § The mean end-to-end distance is: § For the freely jointed (uncorrelated) chain, the second (cross) term of the equation is 0. Thus <r 2>=Na 2, or Dr~N 1/2 (|r|=0) § The overall size of a random walk is proportional to the square root of the number of steps

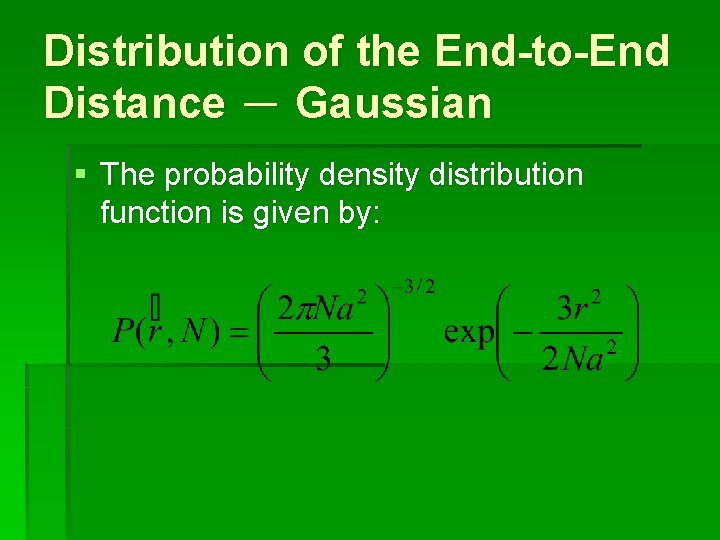
Distribution of the End-to-End Distance - Gaussian § The probability density distribution function is given by:

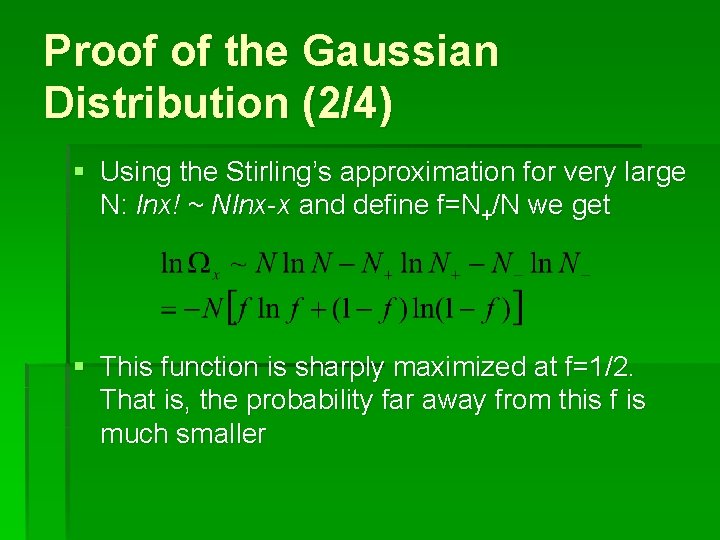
Proof of the Gaussian Distribution (1/4) § Consider a walk in one dimension first: ax is the step length, N+(N-) is the forward (backward) steps, and total steps N=N++N§ After N steps, the end-to-end distance Rx=(N+-N-)ax § The probability of this Rx is given by:

Proof of the Gaussian Distribution (2/4) § Using the Stirling’s approximation for very large N: lnx! ~ Nlnx-x and define f=N+/N we get § This function is sharply maximized at f=1/2. That is, the probability far away from this f is much smaller

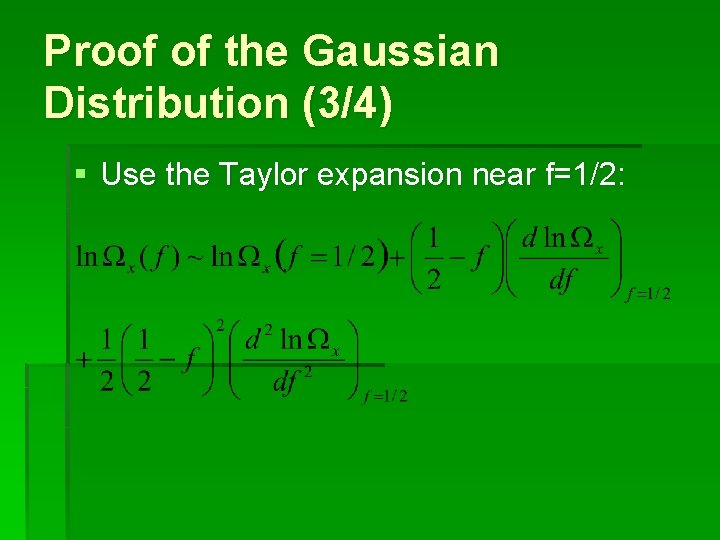
Proof of the Gaussian Distribution (3/4) § Use the Taylor expansion near f=1/2:

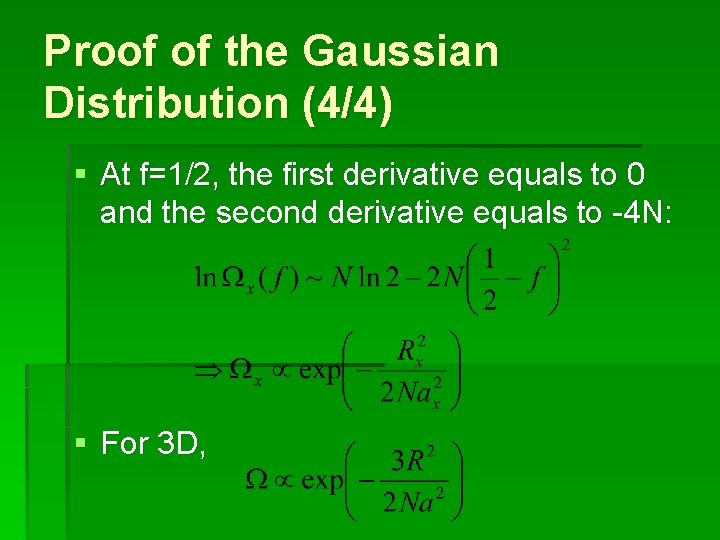
Proof of the Gaussian Distribution (4/4) § At f=1/2, the first derivative equals to 0 and the second derivative equals to -4 N: § For 3 D,

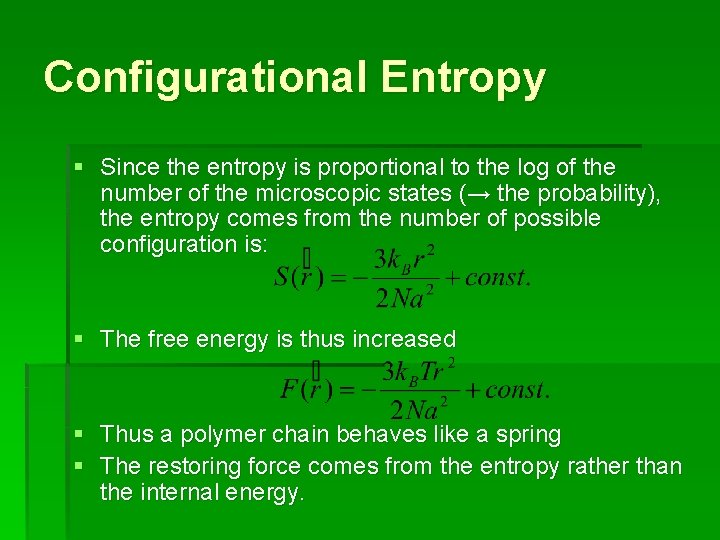
Configurational Entropy § Since the entropy is proportional to the log of the number of the microscopic states (→ the probability), the entropy comes from the number of possible configuration is: § The free energy is thus increased § Thus a polymer chain behaves like a spring § The restoring force comes from the entropy rather than the internal energy.

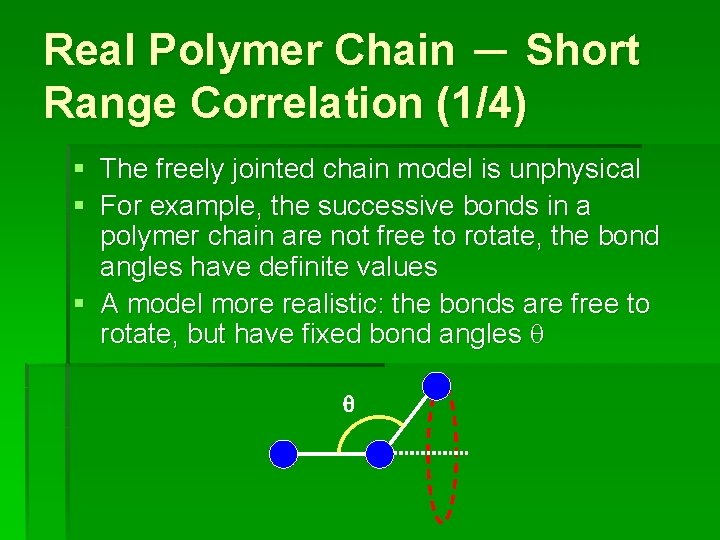
Real Polymer Chain - Short Range Correlation (1/4) § The freely jointed chain model is unphysical § For example, the successive bonds in a polymer chain are not free to rotate, the bond angles have definite values § A model more realistic: the bonds are free to rotate, but have fixed bond angles q q

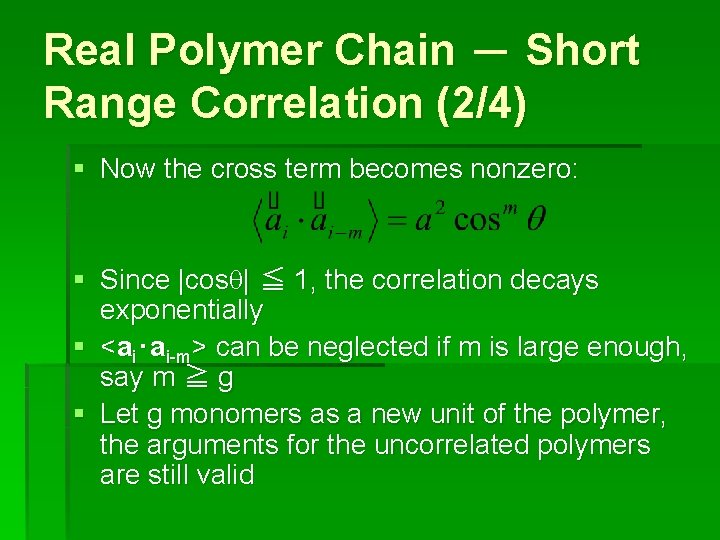
Real Polymer Chain - Short Range Correlation (2/4) § Now the cross term becomes nonzero: § Since |cosq| ≦ 1, the correlation decays exponentially § <ai‧ai-m> can be neglected if m is large enough, say m ≧ g § Let g monomers as a new unit of the polymer, the arguments for the uncorrelated polymers are still valid

Real Polymer Chain - Short Range Correlation (3/4) § Let ci denotes the end-to-end vector of the i-th subunit § Now there is N/g subunits of the polymer § From the free jointed chain model we get: § Here b is an effective monomer size, or the statistical step length § The effect of the correlation can be characterized by the “characteristic ratio”:

Real Polymer Chain - Short Range Correlation (4/4) § From the discussions above we see § The long-range structure (the scaling of the chain dimension with the square root of the degree of N) is given by statistics § This behavior is universal – independent of the chemical details of the polymer § All the effects of the details go into one parameter – the effective bond length § This parameter can be calculated from theory or extracted from experiments

Real Polymer Chain – Excluded Volume § In the previous discussions, interactions between distant monomers are neglected § The simplest interaction: hard core repulsion – no two monomers can occupy the same space at the same time § This is a long-range interaction which may causes long-range correlation of the shape of the chain

Real Polymer Chain – Excluded Volume § There are N monomers in the space with volume V=r 3 § The concentration of the monomers c ~ N/r 3 § If the volume of the monomer is v, the total accessible volume becomes V-Nv

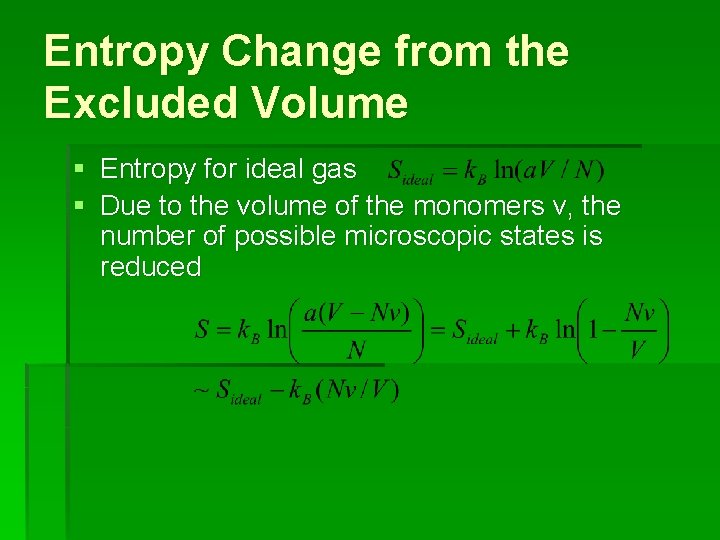
Entropy Change from the Excluded Volume § Entropy for ideal gas § Due to the volume of the monomers v, the number of possible microscopic states is reduced

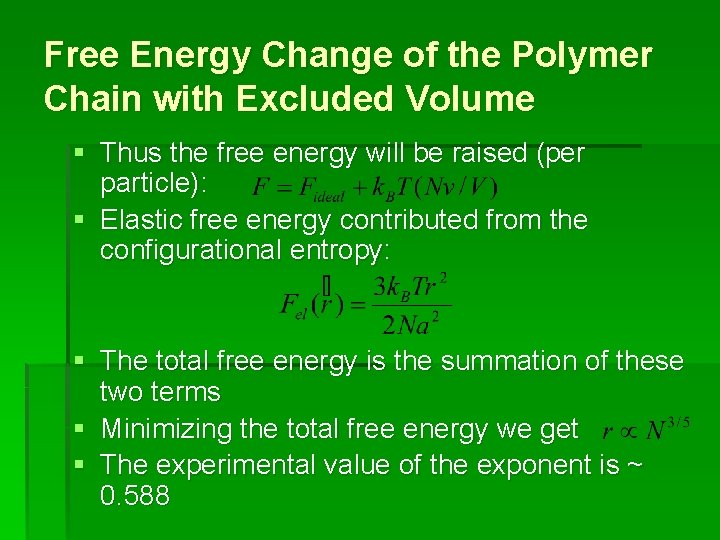
Free Energy Change of the Polymer Chain with Excluded Volume § Thus the free energy will be raised (per particle): § Elastic free energy contributed from the configurational entropy: § The total free energy is the summation of these two terms § Minimizing the total free energy we get § The experimental value of the exponent is ~ 0. 588