Statistical Mechanics and Soft Condensed Matter Amphiphile aggregation

- Slides: 14

Statistical Mechanics and Soft Condensed Matter Amphiphile aggregation: critical micelle concentration by Pietro Cicuta

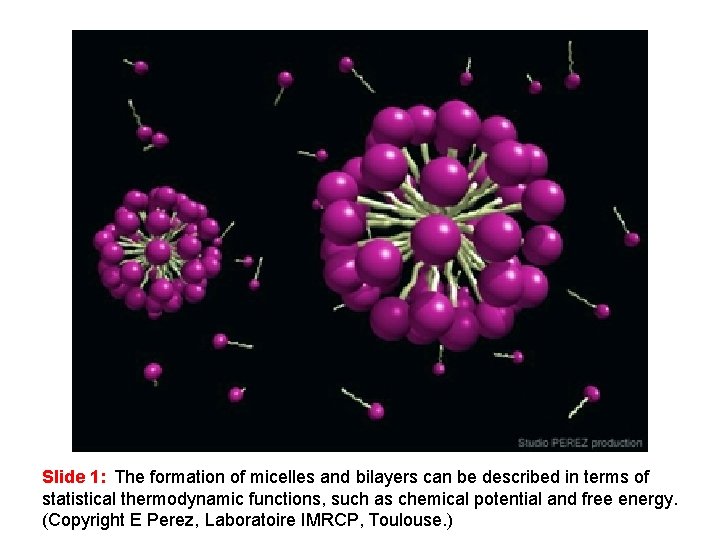
Slide 1: The formation of micelles and bilayers can be described in terms of statistical thermodynamic functions, such as chemical potential and free energy. (Copyright E Perez, Laboratoire IMRCP, Toulouse. )

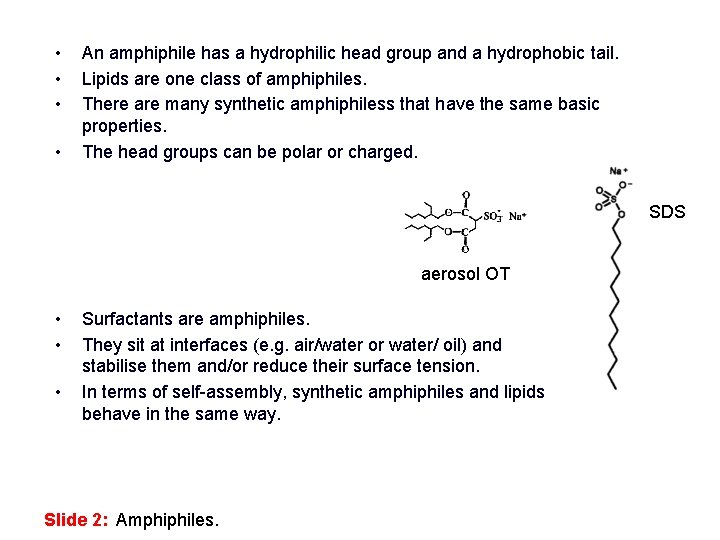
• • An amphiphile has a hydrophilic head group and a hydrophobic tail. Lipids are one class of amphiphiles. There are many synthetic amphiphiless that have the same basic properties. The head groups can be polar or charged. SDS aerosol OT • • • Surfactants are amphiphiles. They sit at interfaces (e. g. air/water or water/ oil) and stabilise them and/or reduce their surface tension. In terms of self-assembly, synthetic amphiphiles and lipids behave in the same way. Slide 2: Amphiphiles.

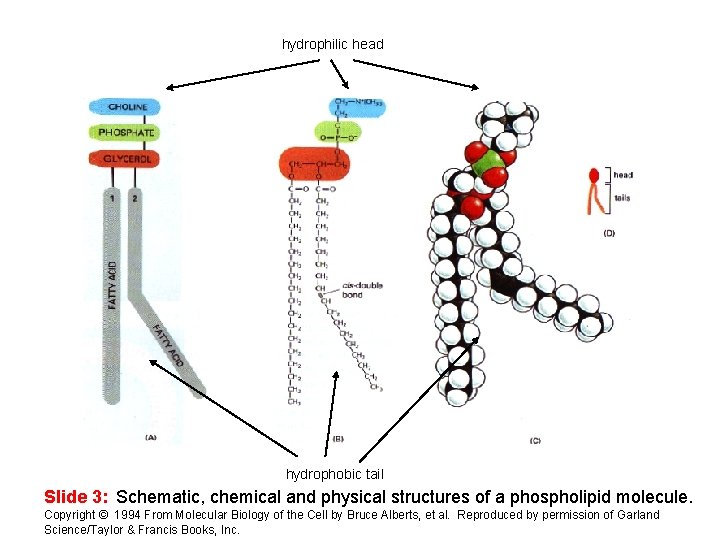
hydrophilic head hydrophobic tail Slide 3: Schematic, chemical and physical structures of a phospholipid molecule. Copyright © 1994 From Molecular Biology of the Cell by Bruce Alberts, et al. Reproduced by permission of Garland Science/Taylor & Francis Books, Inc.

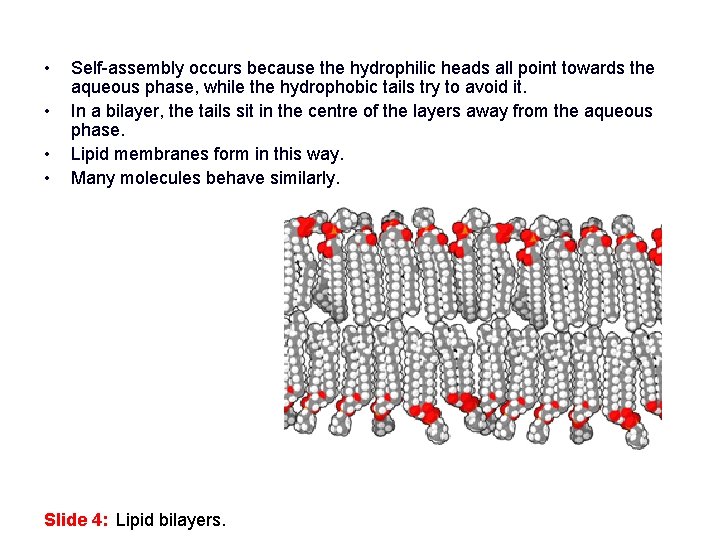
• • Self-assembly occurs because the hydrophilic heads all point towards the aqueous phase, while the hydrophobic tails try to avoid it. In a bilayer, the tails sit in the centre of the layers away from the aqueous phase. Lipid membranes form in this way. Many molecules behave similarly. Slide 4: Lipid bilayers.

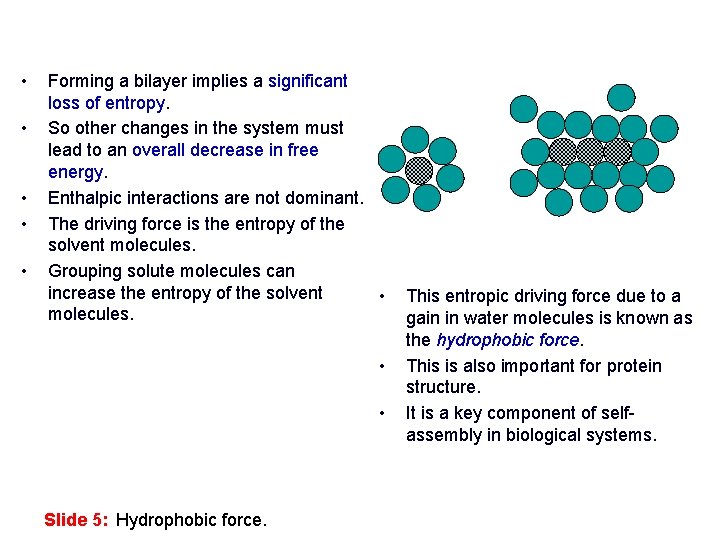
• • • Forming a bilayer implies a significant loss of entropy. So other changes in the system must lead to an overall decrease in free energy. Enthalpic interactions are not dominant. The driving force is the entropy of the solvent molecules. Grouping solute molecules can increase the entropy of the solvent • molecules. • • Slide 5: Hydrophobic force. This entropic driving force due to a gain in water molecules is known as the hydrophobic force. This is also important for protein structure. It is a key component of selfassembly in biological systems.

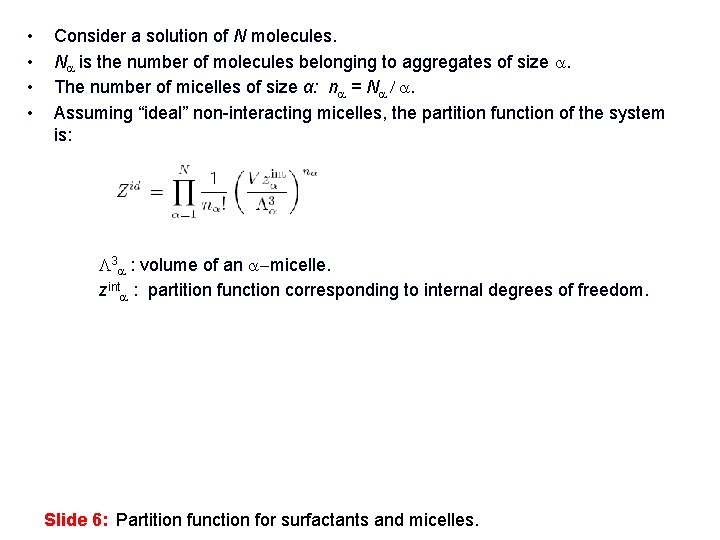
• • Consider a solution of N molecules. Na is the number of molecules belonging to aggregates of size a. The number of micelles of size α: na = Na / a. Assuming “ideal” non-interacting micelles, the partition function of the system is: L 3 a : volume of an a-micelle. zinta : partition function corresponding to internal degrees of freedom. Slide 6: Partition function for surfactants and micelles.

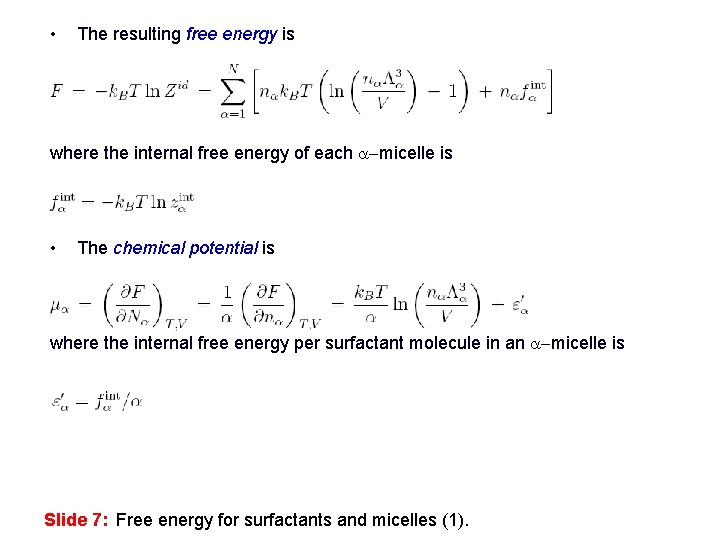
• The resulting free energy is where the internal free energy of each a-micelle is • The chemical potential is where the internal free energy per surfactant molecule in an a-micelle is Slide 7: Free energy for surfactants and micelles (1).

• • • Change variable to the molar fraction. Now xa is the molar fraction of the molecules in any a-micelle. In terms of the molar fractions, the chemical potential is where the free energy change of putting a molecule from the bulk into an a-micelle is and the mean volume of molecules in solution is v = V/N. Slide 8: Free energy for surfactants and micelles (2).

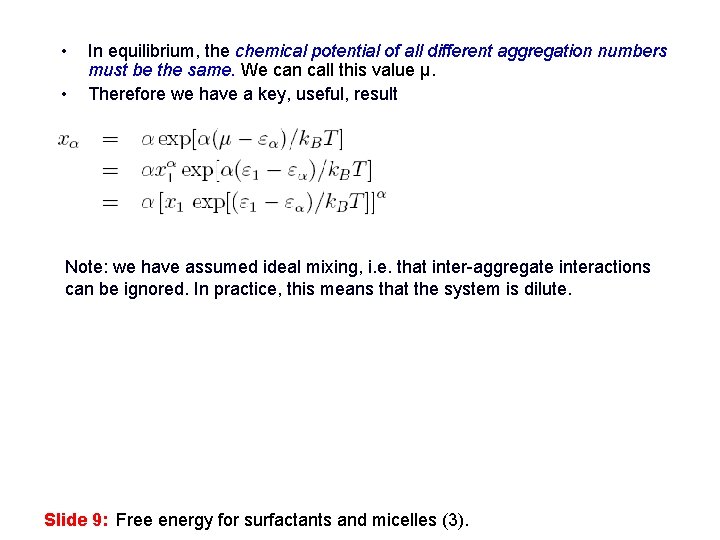
• • In equilibrium, the chemical potential of all different aggregation numbers must be the same. We can call this value μ. Therefore we have a key, useful, result Note: we have assumed ideal mixing, i. e. that inter-aggregate interactions can be ignored. In practice, this means that the system is dilute. Slide 9: Free energy for surfactants and micelles (3).

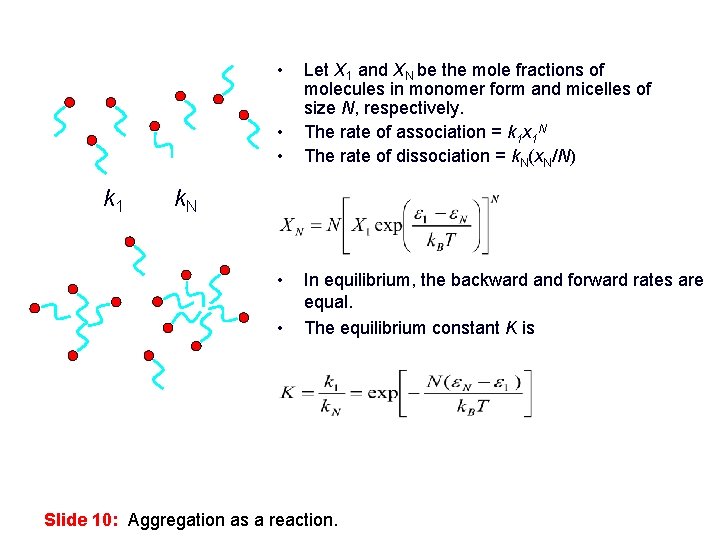
• • • k 1 k. N • • Let X 1 and XN be the mole fractions of molecules in monomer form and micelles of size N, respectively. The rate of association = k 1 x 1 N The rate of dissociation = k. N(x. N/N) In equilibrium, the backward and forward rates are equal. The equilibrium constant K is Slide 10: Aggregation as a reaction.

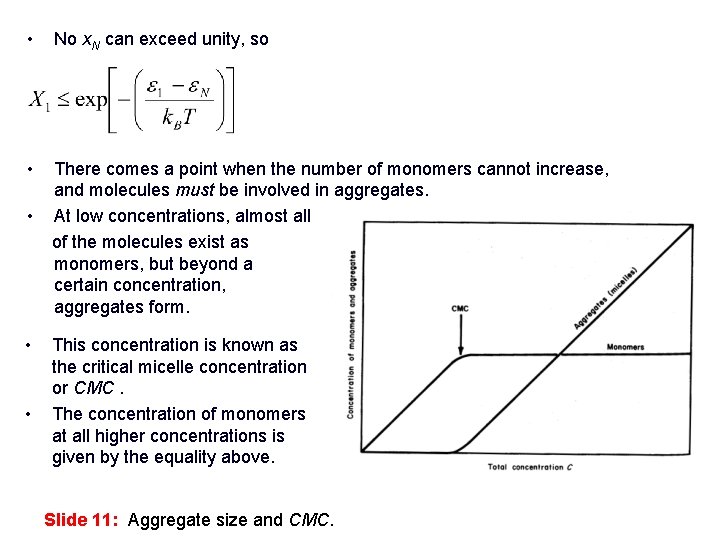
• No x. N can exceed unity, so • There comes a point when the number of monomers cannot increase, and molecules must be involved in aggregates. • At low concentrations, almost all of the molecules exist as monomers, but beyond a certain concentration, aggregates form. • • This concentration is known as the critical micelle concentration or CMC. The concentration of monomers at all higher concentrations is given by the equality above. Slide 11: Aggregate size and CMC.

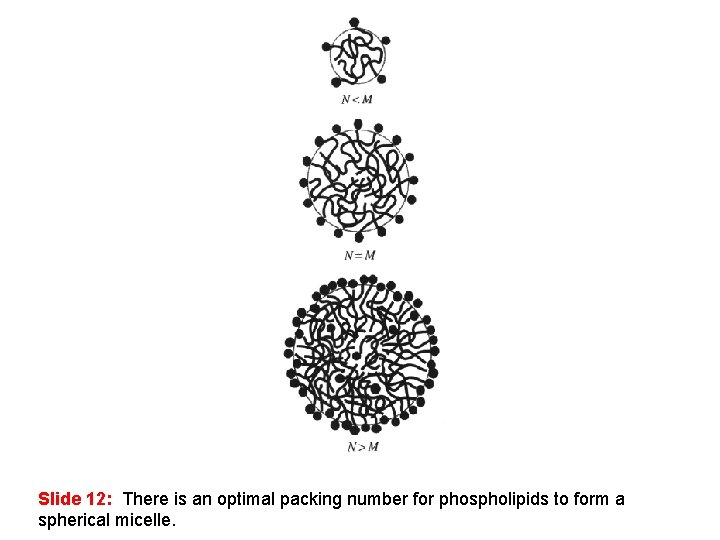
Slide 12: There is an optimal packing number for phospholipids to form a spherical micelle.

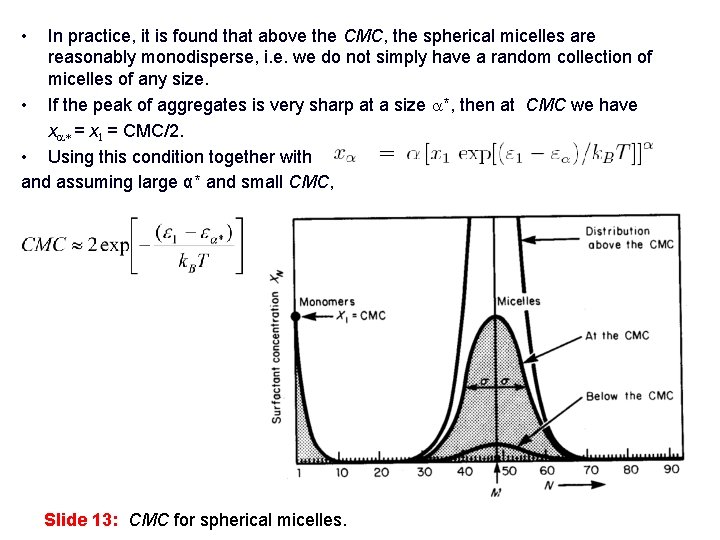
• In practice, it is found that above the CMC, the spherical micelles are reasonably monodisperse, i. e. we do not simply have a random collection of micelles of any size. • If the peak of aggregates is very sharp at a size a*, then at CMC we have xa* = x 1 = CMC/2. • Using this condition together with and assuming large α* and small CMC, Slide 13: CMC for spherical micelles.