Introduction to Thermostatics and Statistical Mechanics A typical





- Slides: 15

Introduction to Thermostatics and Statistical Mechanics • A typical physical system has NA = 6. 023 X 1023 particles. • Each particle has 3 positions and 3 momentums. • Thus there around 1024 degrees of freedom • But we see that in day to day life, we do not need to specify that many variables • For example we need to specify only temperature (T), volume (V), and number of moles (N) of the gas in the room and we specify the system completely. • This is almost a miracle. The reason for this miracle is that: • We are interested in processes that are slow on time-scales of atomic vibrations and occur at larger length scales than the atomic spacing. The symmetries of most system work in such a way that of the 1024 degrees of freedom we are left with only a few variables. • The 1024 variables are called microvariables. • The variables (for example, T, V, N) are called macrovariables.

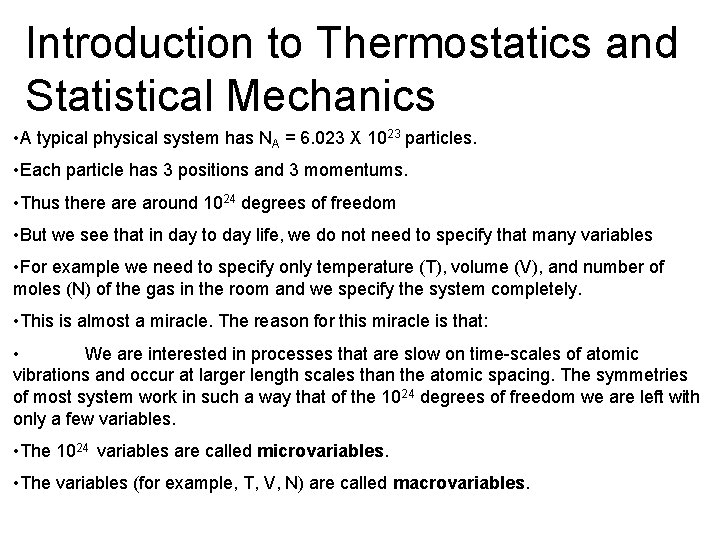
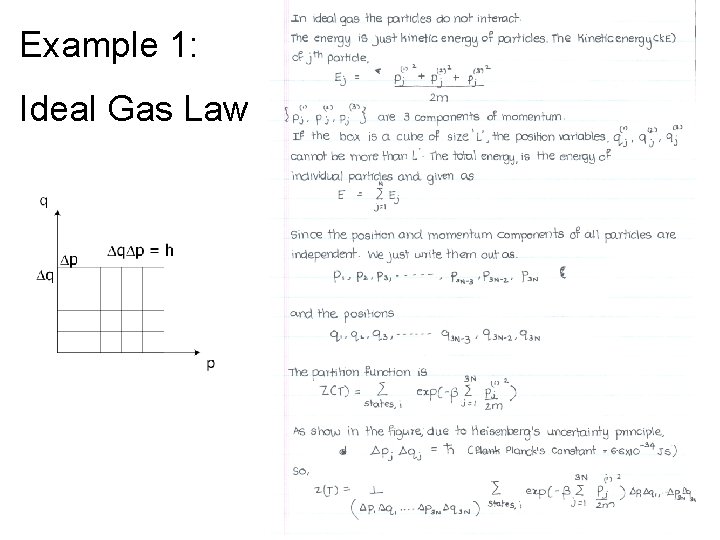
Microstates and Macrostates Example 1: Gas in a Box • Described by microstate {pi, qi}. The position and momentum of all particles. • 3 positions, qi and 3 momentums, pi per particle. So in all (3+3) X 1023 microvariables. • Macrostate described by 3 quantities T, V, N.

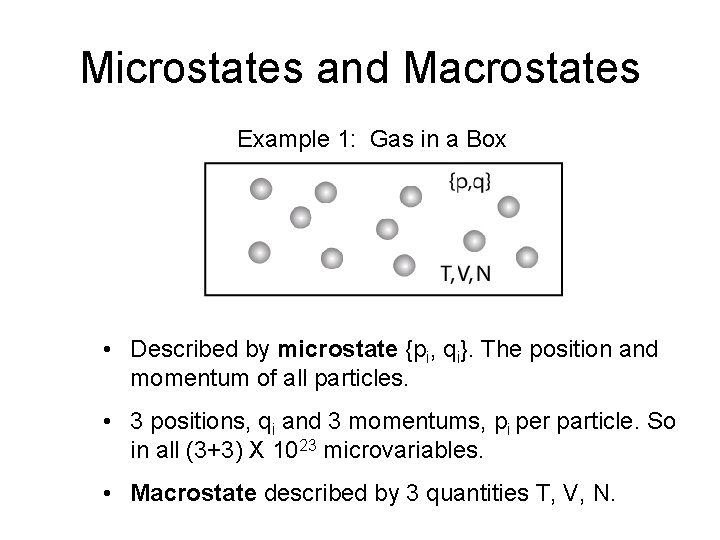
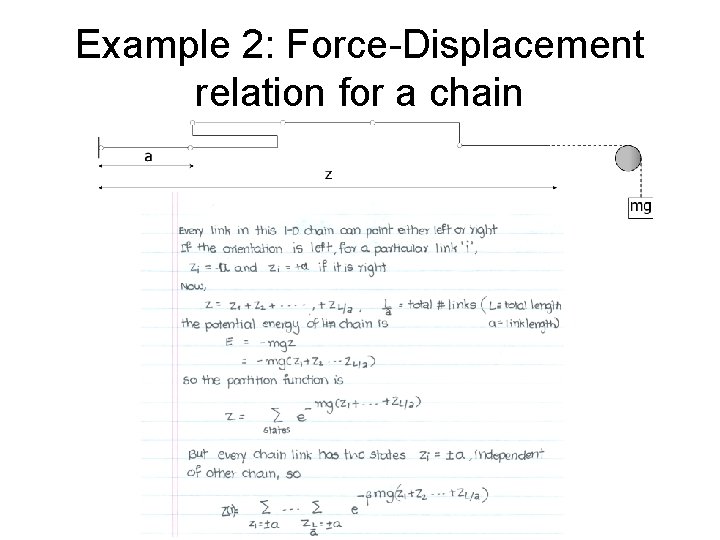
Microstates and Macrostates Example 2: Polymer Chain • The microstate described a particular configuration of the chain on the lattice. • If there are N links on the chain, there are N variables. • Macrostate described by just thermodynamic radius R of the chain.

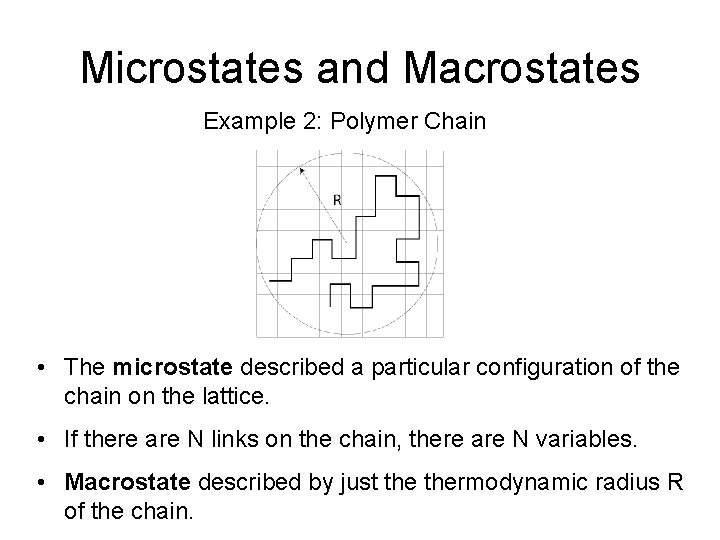
Microstates and Macrostates Example 3: Gas Adsorption • The gas binds on the binding sites on the substrate with favorable energy. • The microstate described by a particular binding configuration of the gas on the substrate. • Macrostate described by just the average number <N> of particles bound to the substrate.

Goal • There are many microstates. • We get experimentally reproducible behavior from only a few macrovariables. • If that macrovariable is, say, E, define where, pi is the probability that the system is in state i. • We want to find, pi. Given <Ei>.

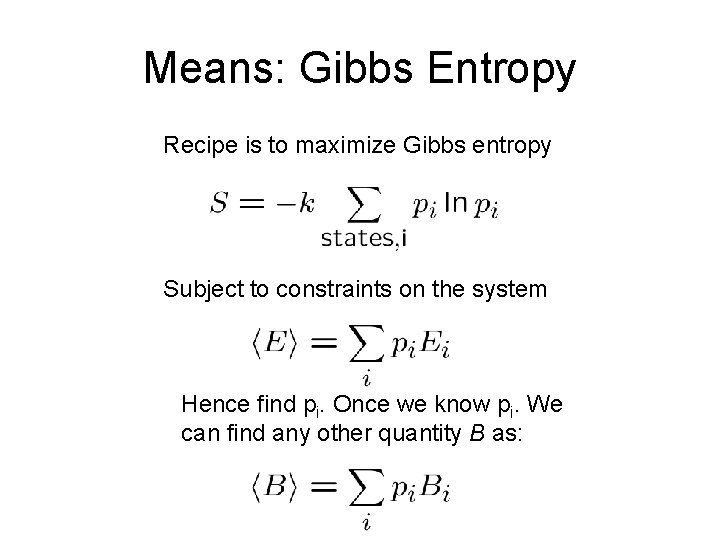
Means: Gibbs Entropy Recipe is to maximize Gibbs entropy Subject to constraints on the system Hence find pi. Once we know pi. We can find any other quantity B as:

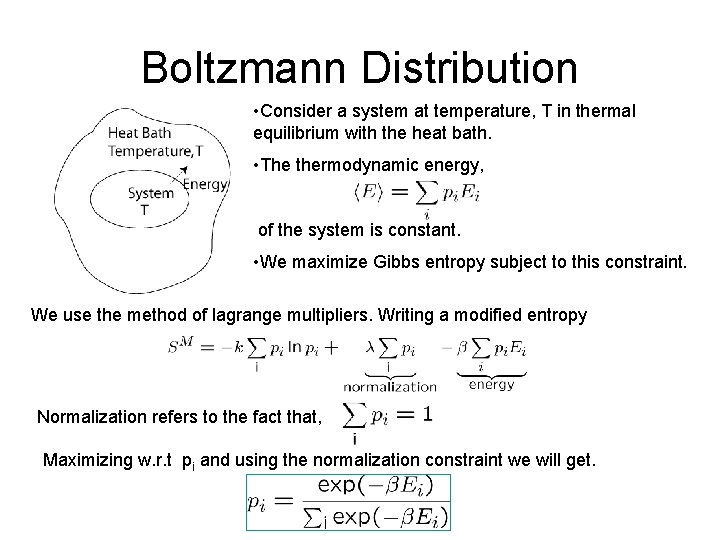
Boltzmann Distribution • Consider a system at temperature, T in thermal equilibrium with the heat bath. • The thermodynamic energy, of the system is constant. • We maximize Gibbs entropy subject to this constraint. We use the method of lagrange multipliers. Writing a modified entropy Normalization refers to the fact that, Maximizing w. r. t pi and using the normalization constraint we will get.

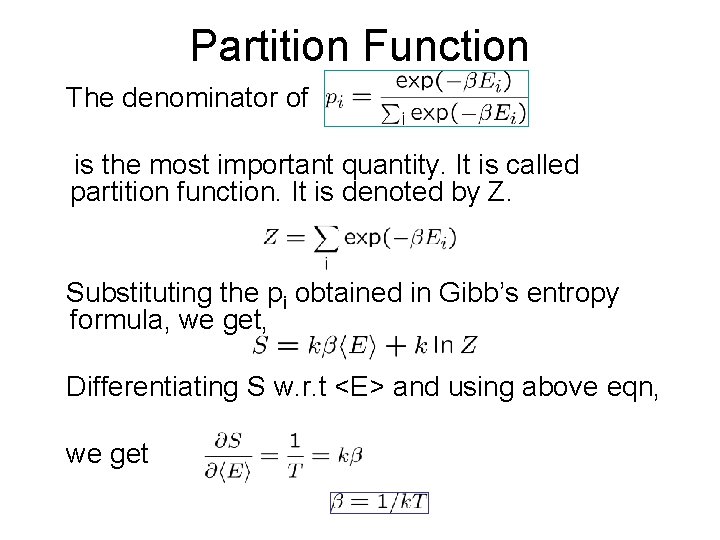
Partition Function The denominator of is the most important quantity. It is called partition function. It is denoted by Z. Substituting the pi obtained in Gibb’s entropy formula, we get, Differentiating S w. r. t <E> and using above eqn, we get

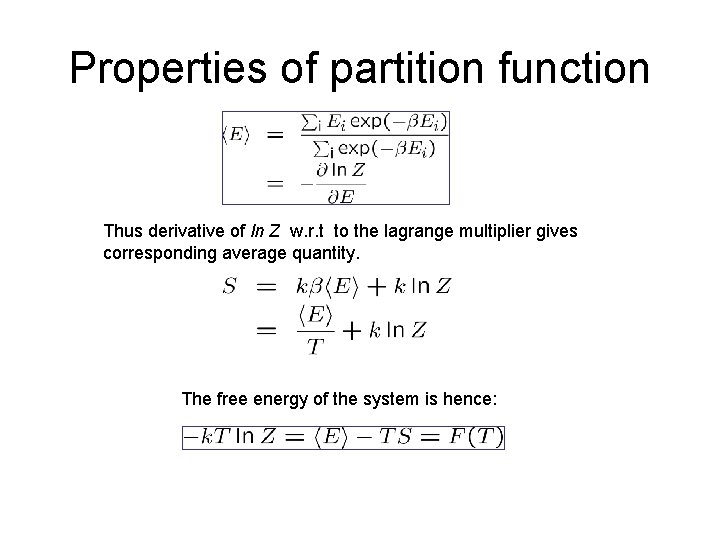
Properties of partition function Thus derivative of ln Z w. r. t to the lagrange multiplier gives corresponding average quantity. The free energy of the system is hence:

Example 1: Ideal Gas Law



Example 2: Force-Displacement relation for a chain

