Polio and Polio Vaccine Epidemiology and Prevention of












- Slides: 17

Polio and Polio Vaccine Epidemiology and Prevention of Vaccine. Preventable Diseases National Immunization Program Centers for Disease Control and Prevention Revised January 2007

NOTICE Content and order of the slides in this file may differ from those presented on the broadcast and webcast

Poliovirus • Enterovirus (RNA) • Three serotypes: 1, 2, 3 • Minimal heterotypic immunity • • between serotypes Infection is acquired through the mouth Most poliovirus infections are asymptomatic

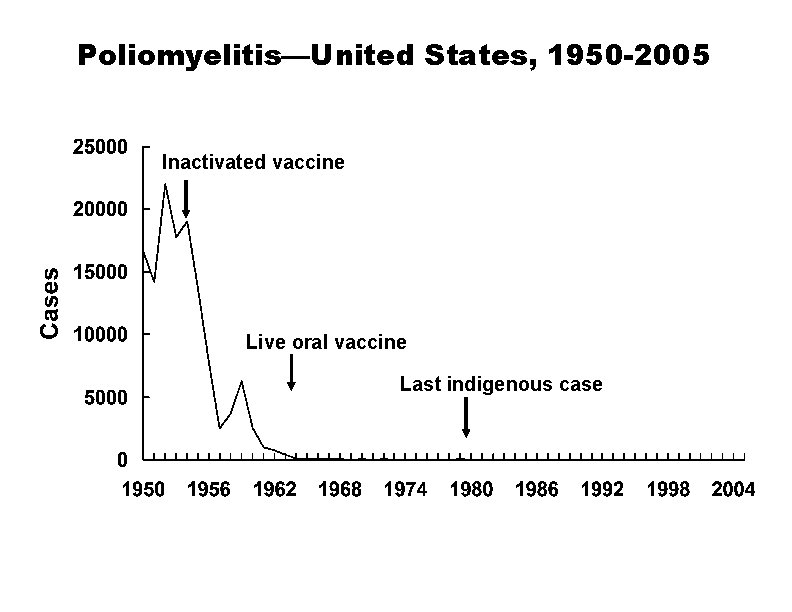
Poliomyelitis—United States, 1950 -2005 Inactivated vaccine Live oral vaccine Last indigenous case

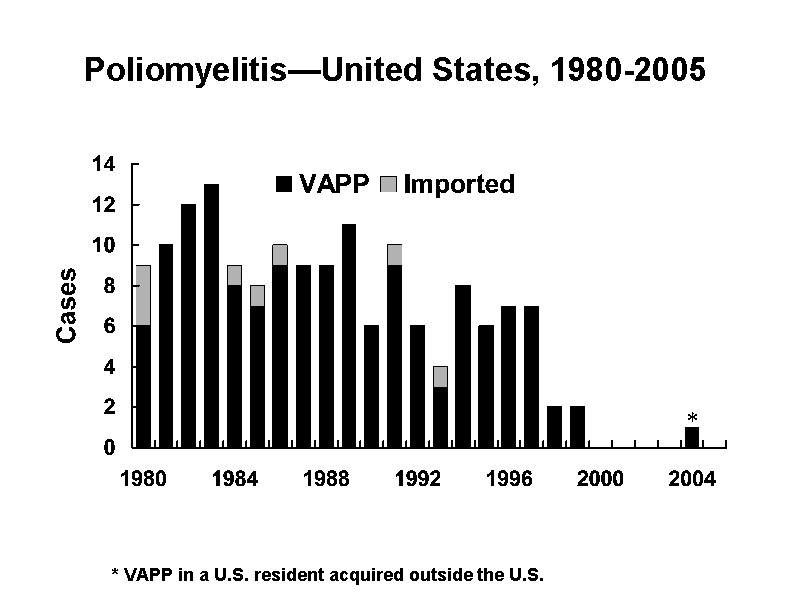
Poliomyelitis—United States, 1980 -2005 * * VAPP in a U. S. resident acquired outside the U. S.

Vaccine-Derived Poliovirus Infections-MN, 2005 • 7 month old infant with a severe • • • immunodeficiency Infected with a type 1 poliovirus derived from the vaccine strain 7 additional infections in the community None of the infected children were paralyzed or had other symptoms

Poliovirus Vaccine • • • 1955 Inactivated vaccine 1961 Types 1 and 2 monovalent OPV 1962 Type 3 monovalent OPV 1963 Trivalent OPV 1987 Enhanced-potency IPV (IPV) 2000 OPV discontinued in U. S.

Inactivated Polio Vaccine • Highly effective in producing • • immunity to poliovirus >90% immune after 2 doses >99% immune after 3 doses

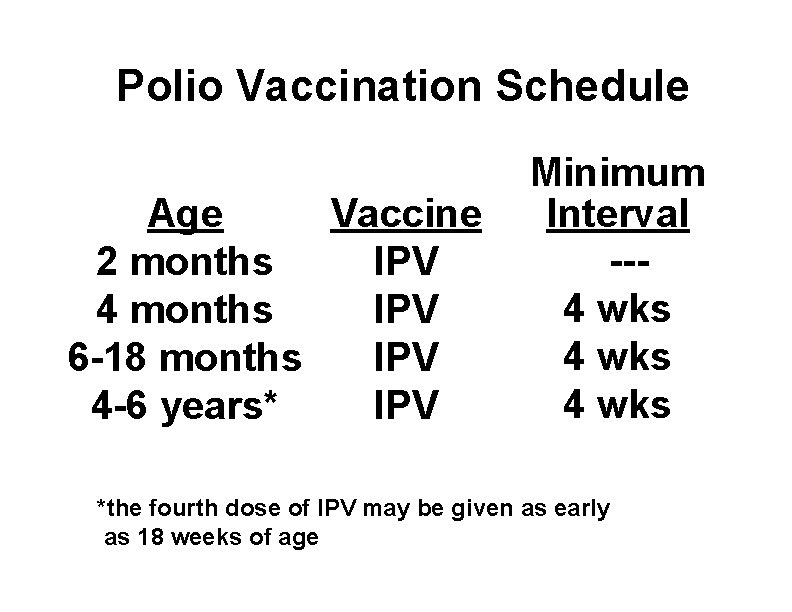
Polio Vaccination Schedule Age Vaccine 2 months IPV 4 months IPV 6 -18 months IPV 4 -6 years* IPV Minimum Interval --4 wks *the fourth dose of IPV may be given as early as 18 weeks of age

Schedules that Include Both IPV and OPV • Only IPV is available in the • • United States Schedule begun with OPV should be completed with IPV Any combination of 4 doses of IPV and OPV by 5 years constitutes a complete series

Polio Vaccination of Adults • Routine vaccination of U. S. residents • 18 years of age or older not recommended Should be considered are those who travel to polio endemic areas of the world and selected laboratory workers

Polio Vaccination of Unvaccinated Adults • Use IPV • Use standard IPV schedule if • possible (0, 1 -2 months, 6 -12 months) May separate doses by 4 weeks if accelerated schedule needed

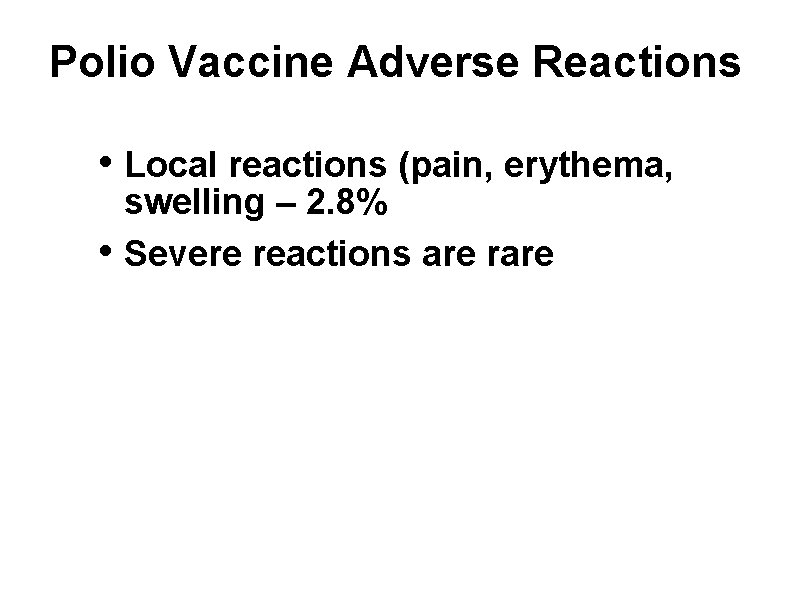
Polio Vaccine Adverse Reactions • Local reactions (pain, erythema, • swelling – 2. 8% Severe reactions are rare

Polio Vaccine Contraindications and Precautions • Severe allergic reaction to a vaccine • component or following a prior dose of vaccine Moderate or severe acute illness

Polio Eradication • Last case in United States in 1979 • last case of wild poliovirus in the • Western Hemisphere occurred in Peru in 1991 World Health Organization established a polio eradication program in 1988

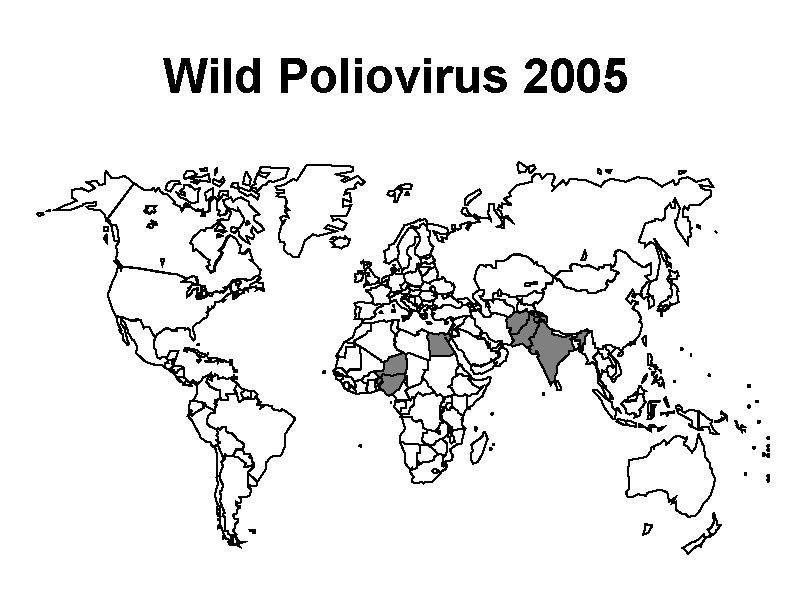
Wild Poliovirus 2005

National Immunization Program Contact Information • Telephone 800. CDC. INFO • Email nipinfo@cdc. gov • Website www. cdc. gov/nip
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Opv medical abbreviation
Opv medical abbreviation What is post polio syndrome
What is post polio syndrome Introduction of immunization
Introduction of immunization The most effective vaccine yet
The most effective vaccine yet Kernig ve brudzinski
Kernig ve brudzinski Oral-virelon impfung
Oral-virelon impfung Polio
Polio Porto polio
Porto polio Polio
Polio Melano medical term
Melano medical term Transverse myelitis
Transverse myelitis Polio plus
Polio plus Polio patient
Polio patient Difference between descriptive and analytical epidemiology
Difference between descriptive and analytical epidemiology Advantages and disadvantages of nutritional epidemiology
Advantages and disadvantages of nutritional epidemiology Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology Incidence vs prevalence
Incidence vs prevalence