EPIDEMIOLOGY OF POLIO MYELITIS AND POLIO ERADICATION PROGRAMME

















![2005 1, 831 cases of wild poliovirus (excludes vaccine derived polio viruses [8]). 727 2005 1, 831 cases of wild poliovirus (excludes vaccine derived polio viruses [8]). 727](https://slidetodoc.com/presentation_image_h/7e59937f7844bc8c3d8e9bb61254e08b/image-21.jpg)

























- Slides: 51

EPIDEMIOLOGY OF POLIO MYELITIS AND POLIO ERADICATION PROGRAMME IN INDIA DR. I. SELVARAJ, I. R. M. S B. Sc. , M. B. B. S. , (M. D COMMUNITY MEDICINE) D. P. H. , D. I. H. , P. G. C. H&FW(NIHFW, NEW DELHI) Sr. D. M. O (ON STUDY LEAVE) INDIAN RAILWAY MEDICAL SERVICE

• THE AMERICAS WERE CERTIFIED POLIO-FREE IN 1994. (36 COUNTRIES) • THE WESTERN PACIFIC WAS CERTIFIED POLIO -FREE IN 2000. (37 COUNTRIES AND AREAS INCLUDING CHINA) • EUROPE, COMPOSED OF 51 COUNTRIES, WAS CERTIFIED POLIO-FREE IN JUNE 2002. (51 COUNTRIES) • WITH ONLY SIX POLIO ENDEMIC COUNTRIES LEFT IN THE WORLD, POLIO TRANSMISSION COULD BE STOPPED BY END 2005. THE WORLD COULD THEN BE CERTIFIED POLIO-FREE BY END-2008.


HISTORY 1789 - British physician Michael Underwood provides the first clinical description of polio, referring to it as "debility of the lower extremities. " 1840 - German physician Jacob von Heine publishes a 78 -page monograph in 1840 which not only describes the clinical features of the disease, but also notes that its symptoms suggest the involvement of the spinal cord. 1908 - Austrian physicians Karl Landsteiner and Erwin Popper make the first hypothesis that polio may be caused by a virus.

In 1908, Karl Landsteiner & Erwin Popper discovered a filterable agent as the cause of poliomyelitis. An extract of medula from a fatal human case was injected intraperitoneally in monkeys. He worked then at the Pasteur Institute, because no monkeys were available in the University of Vienna. The lesions that appeared were indistinguishable from those found in humans. They could not pass the disease monkey to monkey, but Simon Flexner & Paul Lewis managed this and found antibodies. Arnold Netter & Constantin Levaditti found antibodies in human convalescents in 1908. Levaditti & Landsteiner demonstrated neutralizing antibodies in monkey serum against active virus. Frank Mcfarland Burnet & Jean Mac. Namara en 1931 demonstrated serotypes.

In 1936, Albert Sabin & Peter Olitsky cultured poliovirus in embryonic nervous cells. In 1949, John Enders, Thomas Weller & Frederick C. Robbins grew the virus in muscle cells (fibroblasts) human embryonic skin cells, connective tissue cells, intestine and nervous cells, winning the Nobel prize in 1954. The important production point is that the virus grows in nonnervous cells.

Sabin, Albert: (1906 -93) Pioneering researcher on viruses and viral diseases who developed the oral live-virus vaccine against polio. Sabin's vaccine came to be preferred over the alternative killed-virus vaccine developed by his bitter rival Dr. Jonas Salk. The Sabin vaccine contains harmless attenuated polio virus. Dr. Sabin first showed that polio virus could grow in human nerve tissue outside the human body. Through research on monkeys he discovered how the polio virus entered the human body. It had been widely thought that the virus entered through the respiratory tract. Sabin proved that the virus first invaded the digestive tract and later attacked nerve tissue. Albert Bruce Sabin was born in Bialystok, Poland. He immigrated with his family to the US in 1921. He graduated from New York University medical school. He trained in pathology, surgery and internal medicine at Bellevue Hospital in New York and spent a year in research at the Lister Institute in London. In 1935 he returned to New York to join the Rockefeller Institute and then in 1939 moved to the University of Cincinnati and its Children's Hospital Research Foundation.


AGENT: POLIO VIRUS The virion consists of a single strand of RNA containing genetic information and a protein coat. Humans are its only natural host. - The poliovirus is a member of a larger family known as Picornaviruses, which also includes rhinoviruses (such as influenza) and the hepatitis A virus. - Polio belongs to the enterovirus subgroup, made up of over 70 viruses that infect the intestines. - It is one of the smallest RNA viruses, measuring around 25 nm in diameter.

EPIDEMIOLOGY • • • AGENT: POLIOVIRUS TYPE : THREE SERO TYPES(TYPE-1, TYPE-2, TYPE-3) RESERVOIR: MAN INFECTIOUS MATERIAL: FAECES, ORO-PHARYNGEAL SECRETIONS INCUBATION PERIOD: 7 TO 14 DAYS( 3 - 35 DAYS) PERIOD OF COMMUNICABILITY: 7 TO 10 DAYS HOST : AGE : 6 MONTHS TO 3 YEARS ENVIRONMENT : RAINY SEASON (JUNE TO SEPTEMBER) MODE OF TRANSMISSION: FAECO – ORAL ROUTE, DROPLET INFECTION

Group: Group IV ((+)ss. RNA) Family: Picornaviridae Genus: Enterovirus Species: Poliovirus


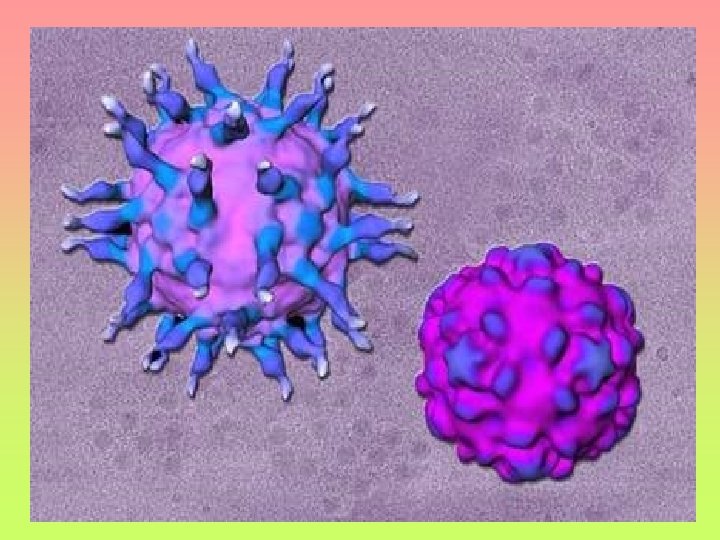
Left: Picture of poliovirus. The poliovirus is extremely small, about 50 nm (nanometer = one-billionth of a meter) Courtesy of David Belnap and James Hogle Right: Cross-section of the poliovirus showing the RNA, capsid, and nerve cell receptors Illustration courtesy of Link Studio

• Inapperent(sub-clinical) Infection: this occurs approximately in 95 per cent of poliovirus infection. There are no presenting symptoms. Recognition only by isolation. • Abortive Polio Or Minor Illness: occurs approximately in 4 -8 per cent of the infection. It causes only a mild or self limiting illness due to viraemia. The patient recovers quickly. • Non paralytic polio: occurs approximately in one per cent of all infections. The presenting features are stiffness and pain in neck and back. The disease lasts for two to ten days. Recovery is rapid. • Paralytic polio: occurs in less then one per cent of infections. The virus enters the brain and causes varying degree of disability.

"Poliomyelitis" comes from the Greek word for gray, polio, and myelo, meaning spinal cord. The Latin suffix itis refers to inflammatory diseases. Among children who are paralyzed by polio: 30% make a full recovery • 30% are left with mild paralysis • 30% have medium to severe paralysis • 10% die

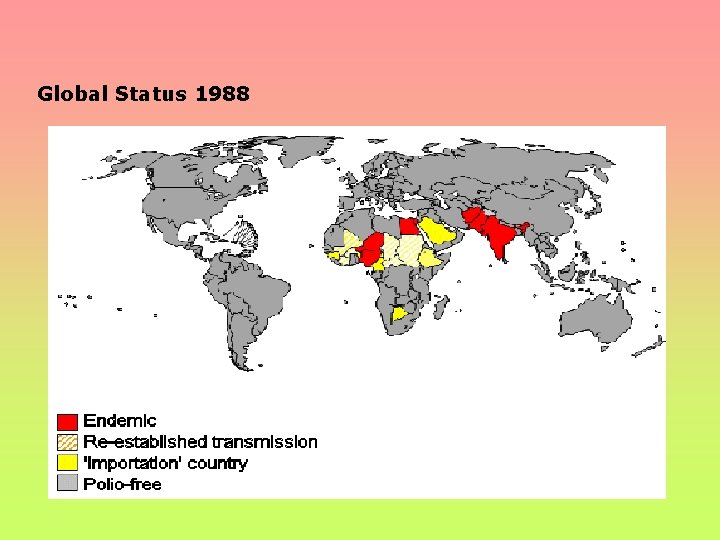
Global Status 1988


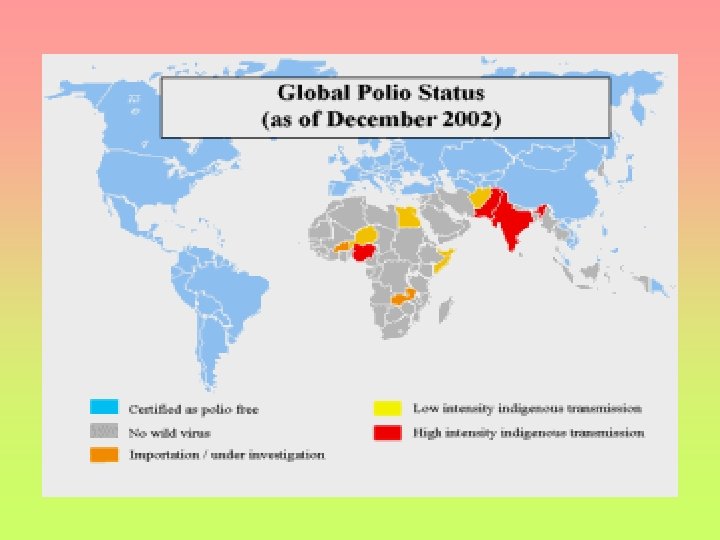
GLOBAL STATUS 2004

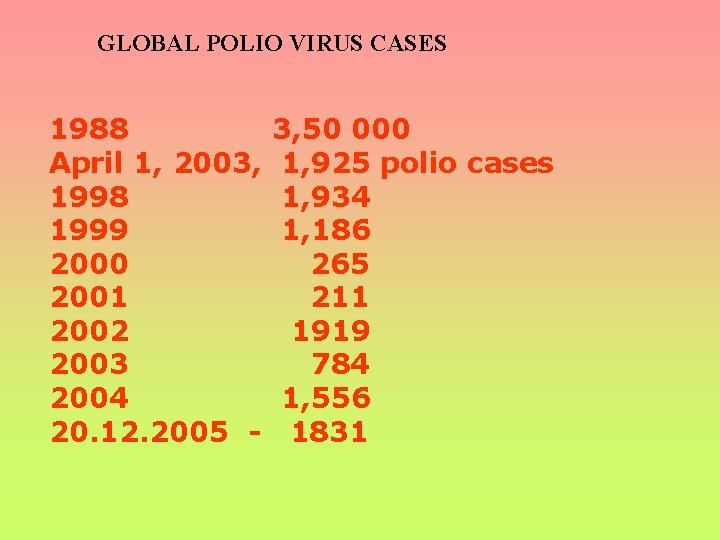
GLOBAL POLIO VIRUS CASES 1988 April 1, 2003, 1998 1999 2000 2001 2002 2003 2004 20. 12. 2005 - 3, 50 000 1, 925 polio cases 1, 934 1, 186 265 211 1919 784 1, 556 1831

![2005 1 831 cases of wild poliovirus excludes vaccine derived polio viruses 8 727 2005 1, 831 cases of wild poliovirus (excludes vaccine derived polio viruses [8]). 727](https://slidetodoc.com/presentation_image_h/7e59937f7844bc8c3d8e9bb61254e08b/image-21.jpg)
2005 1, 831 cases of wild poliovirus (excludes vaccine derived polio viruses [8]). 727 Nigeria (endemic) 478 Yemen (importation) 299 Indonesia (importation) 154 Somalia (importation) 64 India (endemic) 27 Pakistan (endemic) 27 Sudan (re-established transmission) 20 Ethiopia (importation) 9 Angola (importation) 9 Niger (endemic) 7 Afghanistan (endemic) 4 Nepal (importation) 3 Mali (importation) 1 Chad (reestablished transmission) 1 Eritrea (importation) 1 Cameroun (importation) Source: Polio cases from 1 January 2005, as of 17 January 2006

• 25 million children are born in India every year. • There is interval of 11 months between two PPIs. • Over emphasis and too-frequent IPPI rounds and other supplemental immunization activities left a grass root health worker completely exhausted and fatigued. • High population densities, poor sanitation, and low routine immunization coverage. • Resistance for OPV immunization amongst Muslim community. OPV is an anti-fertility vaccine and would lead to impotence in male children or infect them with AIDS. • Children in western UP from Muslim community have consistently been missed both during SIAs and for routine immunization. • Significantly almost 66% of polio cases have occurred among Muslim children. • A dwindling public involvement, and lack of commitment of all sectors of local administration have hampered the progress of this mass-campaign in the most populous and political sensitive states of north India.

In 1993, Kerala became the first state in India to conduct statewide immunization day. In 1994 Tamil Nadu became the second state to conduct statewide immunization day. Delhi became third state, conducting statewide immunization day on 2 nd October 1994 and 4 th December 1994. First PPI held in 1995 -96 all children below 3 years of age were targeted on 9 th December 1995 and 20 th January 1996.

POLIO ERADICATION PROGRAMME • Conduct pulse polio immunisation for two days every year for three to four years or until polio is eradicated. • Sustain high level of routine immunisation. • Monitor OPV coverage at district levels and below. • Improve surveillence capable of detecting all cases of polio. • Ensure rapid case investigation, including the collection of stool samples. • Arrange follow-up of all cases of paralytic polio at 60 days to check for residual paralysis. • Conduct outbreak control for cases confirmed or suspected to stop transmission.

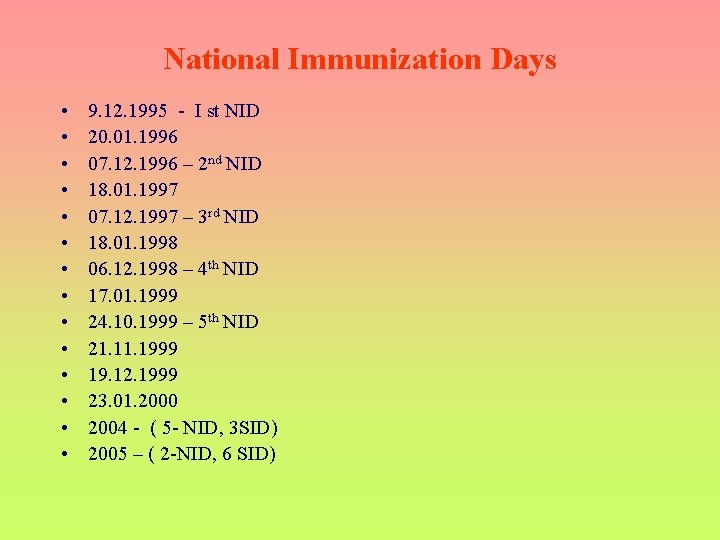
National Immunization Days • • • • 9. 12. 1995 - I st NID 20. 01. 1996 07. 12. 1996 – 2 nd NID 18. 01. 1997 07. 12. 1997 – 3 rd NID 18. 01. 1998 06. 12. 1998 – 4 th NID 17. 01. 1999 24. 10. 1999 – 5 th NID 21. 1999 19. 12. 1999 23. 01. 2000 2004 - ( 5 - NID, 3 SID) 2005 – ( 2 -NID, 6 SID)


GOAL To assist governments in their efforts to immunize every child against polio until polio transmission has stopped, so that the world can be certified polio-free.



From 1996 -97 to all children under the age of 5 years were covered. Till 1998 -99, the PPI Programme consisted of vaccination of children at fixed booths on two National Immunization Days(NID), separated by six weeks, during the winter season. The strategy for 2000– 2001 has been firmed up after studying the epidemiological pattern of the disease in different parts of the country and in consultation with group of national / international experts, specially constituted by WHO at the country’s request. their advice is to adopt a differential approach in response to the varying levels of success already achieved in the different States.

The country has accordingly been divided into three zones : Low Burden Zone (LBZ), Middle Burden Zone (MBZ) and High Burden Zone (HBZ). Experts have suggested that there should be two national immunization days in the months of December 2000 and January 2001, preceded by one Sub-National Immunization Day for 11 States in the month of November 2000 and another SNID for the 4 States of UP, Bihar, West Bengal and Delhi in the month of September 2000, which are in the HBZ zone. The experts also advised that the house to house component need not be insisted on the LBZ areas, while the MBZ and HBZ should continue with the house to house search and immunization programme, as some children in these States are missing vaccination in the NIDs. As regards the LBZ and MBZ areas, experts have advised mop up vaccination around each case of confirmed polio not only in the district in which the case appears but also in the surrounding districts.

The Global Polio Eradication Initiative

OBJECTIVES: • TO INTERRUPT TRANSMISSION OF THE WILD POLIOVIRUS AS SOON AS POSSIBLE AND CERTIFY ALL WHO REGIONS POLIO-FREE BY THE END OF 2005; • TO IMPLEMENT THE POLIO ENDGAME PROGRAMME OF WORK, INCLUDING CONTAINMENT OF WILD POLIOVIRUS, GLOBAL POLIO-FREE CERTIFICATION, AND THE DEVELOPMENT OF A POST-ERADICATION IMMUNIZATION POLICY; • TO CONTRIBUTE TO HEALTH SYSTEMS DEVELOPMENT BY STRENGTHENING ROUTINE IMMUNIZATION AND SURVEILLANCE FOR COMMUNICABLE DISEASES.

Strategies: • HIGH INFANT IMMUNIZATION COVERAGE WITH FOUR DOSES OF ORAL POLIO VACCINE IN THE FIRST YEAR OF LIFE; • SUPPLEMENTARY DOSES OF ORAL POLIO VACCINE TO ALL CHILDREN UNDER FIVE YEARS OF AGE DURING NATIONAL IMMUNIZATION DAYS (NIDS); • SURVEILLANCE FOR WILD POLIOVIRUS THROUGH REPORTING AND LABORATORY TESTING OF ALL CASES OF ACUTE FLACCID PARALYSIS (AFP) AMONG CHILDREN UNDER FIFTEEN YEARS OF AGE; • TARGETED “MOP-UP” CAMPAIGNS ONCE WILD POLIOVIRUS TRANSMISSION IS LIMITED TO A SPECIFIC FOCAL AREA.

Before a WHO region can be certified polio-free, three conditions must be satisfied: ( A) AT LEAST THREE YEARS OF ZERO POLIO CASES DUE TO WILD POLIOVIRUS ( B) EXCELLENT CERTIFICATION STANDARD SURVEILLANCE ( C) EACH COUNTRY MUST ILLUSTRATE THE CAPACITY TO DETECT, REPORT AND RESPOND TO “IMPORTED” POLIO CASES. LABORATORY STOCKS MUST BE CONTAINED AND SAFE MANAGEMENT OF THE WILD VIRUS IN INACTIVATED POLIO VACCINE (IPV) MANUFACTURING SITES MUST BE ASSURED BEFORE THE WORLD CAN BE CERTIFIED POLIOFREE.

In THERE ARE FOUR GLOBAL PRIORITIES TO STOP TRANSMISSION OF THE WILD POLIOVIRUS AND OPTIMIZE THE BENEFITS OF POLIO ERADICATION : 1. 1. Substantial external financial resources are required to support the efforts of developing countries to eradicate polio. These financial resources must be secured to purchase oral polio vaccine (OPV), to plan and implement national immunization days and mop-up campaigns, and to cover surveillance and laboratory costs. 2. 2. India is the highest priority country, because it has the highest number of cases in the world (83%), and for the first time in the Initiative’s history, previously polio-free areas were reinfected, as the epidemic in the north Indian state of Uttar Pradesh spread into such Indian states as Gujarat, Rajasthan and West Bengal. 3. 3. As the world is nearing polio-free status, effective surveillance becomes even more important to quickly identify any potential outbreaks and manage them effectively. Achieving certification standard surveillance is also a requirement for certifying the world polio-free. 4. 4. In conjunction with effective surveillance, it is essential that the capacity is in place to rapidly mount massive immunization response campaigns to manage any wild poliovirus importations quickly and efficiently in polio-free areas

FUTURE BENEFITS OF POLIO ERADICATION ONCE POLIO IS ERADICATED, THE WORLD CAN CELEBRATE NOT ONLY THE ERADICATION OF A DISEASE BUT THE DELIVERY OF A GLOBAL PUBLIC GOOD – SOMETHING FROM WHICH EVERY PERSON, REGARDLESS OF RACE, SEX, ETHNICITY, ECONOMIC STATUS OR RELIGIOUS BELIEF, CAN BENEFIT FOR ALL TIME, NO MATTER WHERE THEY LIVE. THE HUMANITARIAN BENEFIT IS TREMENDOUS, AS BETWEEN 2002 AND 2040, OVER TEN MILLION NEW CASES OF POLIO WORLDWIDE WOULD MANIFEST THEMSELVES. ADDITIONALLY, THE SAVINGS OF POLIO ERADICATION ARE POTENTIALLY AS HIGH AS US$ 1. 5 BILLION PER YEAR – FUNDS THAT COULD BE USED TO ADDRESS OTHER PUBLIC HEALTH PRIORITIES.

VACCINE VIAL MONITOR

THE MAGNESIUM CHLORIDE STABILISED VACCINE WILL MAINTAIN ADEQUATE IMMUNOGENICITY FOR 18 MONTHS WHEN KEPT IN A REFRIGERATOR AT +2°C TO +8°C, FOR SIX WEEKS AT +25°C AND FOR THREE DAYS AT +37°C. AT -20°C, ALL FORMULATIONS AND PRESENTATIONS ARE VERY STABLE AND NO LOSS OF POTENCY HAS BEEN OBSERVED OVER A PERIOD OF MORE THAN FIVE YEARS. VACCINES SHOULD BE INSPECTED VISUALLY FOR ANY PARTICULATE MATTER AND/OR OTHER COLORATION PRIOR TO ADMINISTRATION. DUE TO MINOR VARIATION OF ITS PH, POLIO SABIN™ (ORAL) MAY VARY IN COLOUR FROM LIGHT YELLOW TO LIGHT RED. CHANGES OF THE COLOUR OF THE VACCINE WITHIN THESE RANGES DO NOT SIGNIFY DETERIORATION OF THE VACCINE SHOULD BE STORED IN A REFRIGERATOR BETWEEN +2°C AND +8°C OR IN A FREEZER AT -20°C. FREEZING AND THAWING DOES NOT AFFECT THE TITRE OF THE VACCINE. IN ORDER TO PRESERVE OPTIMAL POTENCY OF POLIO SABIN™ (ORAL), EXPOSURE OF THE VACCINE TO AMBIENT (NON-REFRIGERATED) TEMPERATURES SHOULD BE KEPT TO A MINIMUM AND EXPOSURE TO SUNLIGHT SHOULD BE AVOIDED.


The IEAG also made the following recommendations regarding supplementary immunisation schedule and vaccine: • Use of m. OPV 1 in Bihar, UP and neighboring districts of Uttaranchal, Delhi, and Mumbai/ Thane/Raigad during the May 2005 NID. • Given the high probability of ongoing low level type 1 poliovirus transmission, and the risk of further spread of this virus with the onset of the rainy season in June, m. OPV 1 be used in a further round in the full supplementary NID (SNID) area (Bihar, UP and neighboring districts of Uttaranchal, Delhi, and Mumbai/Thane/Raigad). Any areas not covered with m. OPV 1 in the June SNID should use m. OPV 1 in the August SNID. • Trivalent OPV should be used for the SNIDs during the three remaining SNID rounds in 2005 (August, October and November), except in areas where wild poliovirus type 1 persists, in which case m. OPV 1 should be used in appropriate districts for two sequential rounds. • The geographic extent of the four SNIDs should be expanded to include any additional areas or state where a wild poliovirus is isolated. If wild poliovirus type 1 is isolated, m. OPV 1 should be used for at least two rounds in these areas. • Trivalent OPV should be used for SIAs in 2006 -2007 unless wild poliovirus is isolated, in which case m. OPV should be used in at least two sequential rounds in an appropriate area.

Accurate surveillance for polio is essential for eradication. Surveillance systems for polio have been developed under the guidance of the global polio eradication initiative and use a combination of (1) identification of all potential cases of acute flaccid paralysis (AFP), the most obvious manifestation of polio infection and (2) laboratory evaluation of stools from these cases to confirm poliovirus as the cause. Surveillance of cases of acute flaccid paralysis among children less than 15 years of age is a key component for a well functioning polio surveillance system. The surveillance system works through a network of surveillance medical officers, the responsibility of them lies in assisting the health services departments of all states and maintaining a network of acute flaccid paralysis reporting sites and rapidly investigating the cases. AFP is defined as sudden onset of weakness and floppiness in any part of the body in a child less than 15 years of age. In addition, paralysis in a person of any age in whom polio is suspected is also reported. AFP surveillance is used to detect cases of suspected polio to initiate investigation and control measures. Any case meeting the case definition should be investigated and stool specimens collected.

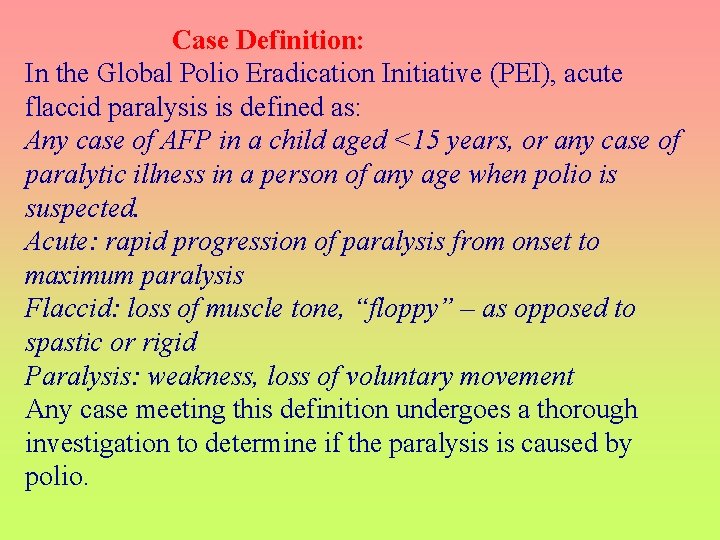
Case Definition: In the Global Polio Eradication Initiative (PEI), acute flaccid paralysis is defined as: Any case of AFP in a child aged <15 years, or any case of paralytic illness in a person of any age when polio is suspected. Acute: rapid progression of paralysis from onset to maximum paralysis Flaccid: loss of muscle tone, “floppy” – as opposed to spastic or rigid Paralysis: weakness, loss of voluntary movement Any case meeting this definition undergoes a thorough investigation to determine if the paralysis is caused by polio.

Components of AFP Surveillance The objective of AFP surveillance is to detect the exact geographic locations where wild polioviruses are circulating in the human population. All cases of acute flaccid paralysis in children aged <15 years are rigorously investigated by a trained medical officer, with collection of stool specimens to determine if poliovirus is the cause of the paralysis. Analysis of the location of polioviruses isolated from AFP cases allows programme managers to plan immunization campaigns (Pulse Polio Immunization) to prevent continuing circulation of virus in these areas.

COMPONENTS OF AFP SURVEILANCE 1. The AFP surveillance network and case notification 2. Case and laboratory investigation 3. Outbreak response and active case search in the community 4. 60 -day follow-up, cross-notification and tracking of cases 5. Data management and case classification 6. Virologic case classification scheme 7. Surveillance performance indicators

The most important aspect of this classification is the collection of 2 adequate stool samples from all cases. Samples are considered adequate if both the specimens (1) are collected within 14 days of paralysis onset and at least 24 hours apart; (2) are of adequate volume (8 -10 g) and (3) arrives at a WHO-accredited laboratory in good condition (ie, no desiccation, no leakage), with adequate documentation and evidence of cold-chain maintenance.



THE GLOBAL POLIO LABORATORY NETWORK IS A 3 -TIER PYRAMIDAL NETWORK OF A TOTAL OF 145 LABORATORIES CLASSIFIED AS NATIONAL, REGIONAL REFERENCE AND GLOBAL SPECIALISED LABORATORIES. THE INDIAN NETWORK IS COMPRISED OF A TOTAL OF EIGHT LABORATORIES WITH ONE GSL AND SEVEN NPL. THESE ARE STRATEGICALLY LOCATED IN DIFFERENT PARTS OF THE COUNTRY FOR QUICK, EASY ACCESS BY THE SURVEILLANCE SYSTEM. A SPECIFIC GEOGRAPHICAL AREA IS SERVED BY EACH LABORATORY. ALL LABORATORIES IN THE GLOBAL POLIO NETWORK FOLLOW STRICT BIO-SAFETY LEVEL 2 FACILITIES. AND ALSO USE (A) IDENTICAL TESTING PROCEDURES AND HIGH QUALITY EQUIPMENT AND REAGENTS, (B) HAVE INSTITUTED QUALITY ASSURANCE PROGRAMMES AND (C) UNDERGO ANNUAL PROFICIENCY TESTING AND ON-SITE EVALUATION BY WHO FOR ACCREDITATION.


Reference: www. who. int www. mohfw. nic. in www. polioeradication. org www. unicef. org/immunization Super course/ Pittsburgh university(www. pitt. edu/~super 1) JIMA DECEMBER 2005 http: //www. polionet. org/vaccine. htm www. npspindia. org
 National leprosy eradication programme ppt
National leprosy eradication programme ppt H pylori eradication therapy
H pylori eradication therapy Weed science definition
Weed science definition Containment eradication
Containment eradication Transverse myelitis physiotherapy
Transverse myelitis physiotherapy Polio
Polio Thesourceagents
Thesourceagents Nutritional epidemiology definition
Nutritional epidemiology definition Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology Incidence and prevalence meaning
Incidence and prevalence meaning Certification board of infection control and epidemiology
Certification board of infection control and epidemiology Epidemiology person place time
Epidemiology person place time Post-polio syndrome
Post-polio syndrome What is polio disease
What is polio disease The most effective vaccine yet
The most effective vaccine yet Kernig bulgusu
Kernig bulgusu Ipv merieux fachinformation
Ipv merieux fachinformation Antigen attacker
Antigen attacker Polio vaccine acronym
Polio vaccine acronym Porto polio
Porto polio Elsie lacks
Elsie lacks Pithyriasis rosea
Pithyriasis rosea Papa lolos
Papa lolos Polio patient
Polio patient Perbedaan or rr dan pr
Perbedaan or rr dan pr Logistic regression epidemiology
Logistic regression epidemiology Attack rate formula
Attack rate formula Cross sectional study advantages and disadvantages
Cross sectional study advantages and disadvantages Attack rate calculation
Attack rate calculation Bibliography of epidemiology
Bibliography of epidemiology Causal vs association
Causal vs association Formula for attack rate
Formula for attack rate Ramboman analysis
Ramboman analysis Web of causation example
Web of causation example Percentage defination
Percentage defination Defination of epidemiology
Defination of epidemiology Epornithic
Epornithic What is descriptive study in epidemiology
What is descriptive study in epidemiology Spurious association
Spurious association Field epidemiology ppt
Field epidemiology ppt Aims of epidemiology
Aims of epidemiology Gordon epidemiology
Gordon epidemiology Epidemiology kept simple
Epidemiology kept simple Diabetic ketoacidosis epidemiology
Diabetic ketoacidosis epidemiology Distribution in epidemiology
Distribution in epidemiology Effect modification epidemiology
Effect modification epidemiology Distribution in epidemiology
Distribution in epidemiology Gate frame epidemiology
Gate frame epidemiology Epidemiology definition
Epidemiology definition Prevalensi adalah
Prevalensi adalah How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor Nutritional epidemiology
Nutritional epidemiology